
Which of the following reactions is called Rosenmund reaction?
a.) Aldehydes are reduced to alcohols.
b.) Acids are converted to acid chlorides.
c.) Alcohols are reduced to hydrocarbons.
d.) Acid chlorides are reduced to aldehydes.
Answer
594k+ views
Hint: Rosenmund reaction involves the conversion of that compound which contains a halogen atom. The product formed after the reaction is the molecule which upon further reduction gives us alcohol.
Complete answer: To find out the correct option, we should know about what is Rosenmund reaction. So, first of all, let’s see this.
Rosenmund reaction is a hydrogenation reaction. Now the question arises what is hydrogenation. Hydrogenation is a process that involves the addition of hydrogen atoms across a double or triple bond. The Rosenmund reaction involves selectively reduction of acyl chloride to an aldehyde.
The Pd metal on $BaS{O_4}$ is called Rosenmund reagent.
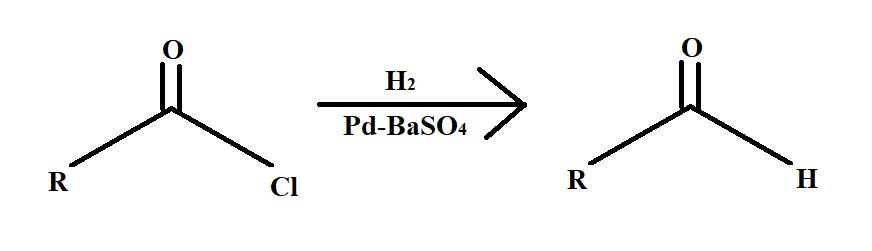
In the above reaction what happens is that a molecule of an acyl chloride is reduced to a molecule of aldehyde in the presence of hydrogen gas over palladium metal surface poisoned by barium sulphate.
So, the correct answer is “Option D”.
Additional Information: Rosenmund catalyst can be prepared by the reduction of palladium(II) chloride solution in the presence of $BaS{O_4}$. The $BaS{O_4}$ has a low surface area which reduces the activity of palladium metal. This helps in prevention of over-reduction.
Note: We can prepare a large number of aldehydes by this method but formaldehyde can-not be prepared by this method because for the formation of formaldehyde; we require formyl chloride which will be reduced to formaldehyde. The formyl chloride is not stable at room temperature. So, this reaction is not possible.
Complete answer: To find out the correct option, we should know about what is Rosenmund reaction. So, first of all, let’s see this.
Rosenmund reaction is a hydrogenation reaction. Now the question arises what is hydrogenation. Hydrogenation is a process that involves the addition of hydrogen atoms across a double or triple bond. The Rosenmund reaction involves selectively reduction of acyl chloride to an aldehyde.
The Pd metal on $BaS{O_4}$ is called Rosenmund reagent.
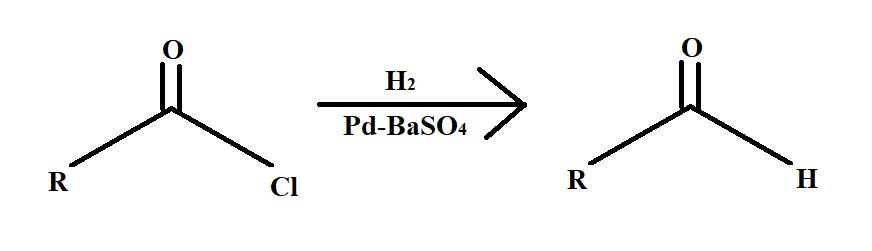
In the above reaction what happens is that a molecule of an acyl chloride is reduced to a molecule of aldehyde in the presence of hydrogen gas over palladium metal surface poisoned by barium sulphate.
So, the correct answer is “Option D”.
Additional Information: Rosenmund catalyst can be prepared by the reduction of palladium(II) chloride solution in the presence of $BaS{O_4}$. The $BaS{O_4}$ has a low surface area which reduces the activity of palladium metal. This helps in prevention of over-reduction.
Note: We can prepare a large number of aldehydes by this method but formaldehyde can-not be prepared by this method because for the formation of formaldehyde; we require formyl chloride which will be reduced to formaldehyde. The formyl chloride is not stable at room temperature. So, this reaction is not possible.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




