
Which of the following reactions are both stereospecific and stereoselective?
This question contains multiple answers.
(a) \[{{S}_{N}}1\]
(b) \[{{S}_{N}}2\]
(c) \[{{E}_{1}}\]
(d) \[{{E}_{2}}\]
Answer
595.2k+ views
Hint: Stereospecific reaction is a reaction which allows formation of only one stereoisomer whereas in the stereoselective reaction two isomers are formed one of them is major and the other is minor.
Complete step by step answer:
Let us study all the reactions one by one:
(a) \[{{S}_{N}}1\]
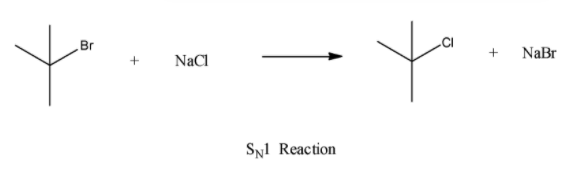
It is non-stereospecific i.e., attacked by nucleophiles which occur from both sides. It has carbocation intermediate and is a unimolecular reaction - rate depends on the concentration of only one reactant.
This reaction gives a racemic mixture.
(b) \[{{S}_{N}}2\]
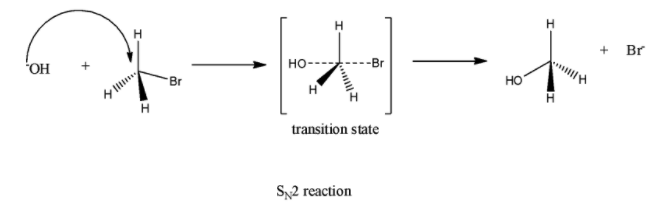
It is a stereospecific reaction because it shows Walden Inversion of configuration. All bonds join and break at the same time. It is a bimolecular reaction - rate depends on the concentration of both the reactants.
This reaction gives enantiomers.
(c) \[{{E}_{1}}\]
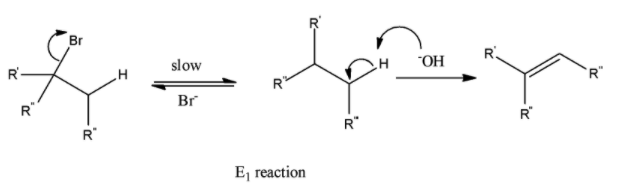
It is a non-stereospecific reaction, as it follows the Saytzeff rule.
It has carbocation as an intermediate.
It is also unimolecular- the rate depends on the concentration of only one reactant.
This reaction gives 2 equal amounts of products.
(d) \[{{E}_{2}}\]
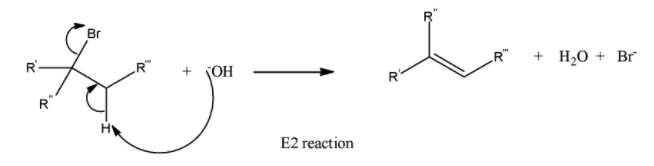
It is a stereospecific reaction because it has Anti and Syn planar geometry. All bonds join and break at the same time. It is also bimolecular- rate depends on the concentration of the reaction.
This reaction is stereoselective- one product is major.
Hence, option (b) and (d) are correct answers.
Additional information:
> \[{{S}_{N}}2\] reactions are always accompanied by inversion of configuration just in the same way as an umbrella turns inside out in a strong wind. This inversion of configuration is called Walden Inversion.
> In \[{{E}_{2}}\], if the halogen is on any carbon atom within the chain, the alkyl halide can undergo dehydrohalogenation in two or more different directions depending upon the number of different types of beta-hydrogen atoms are present. In such cases a more highly substituted product is formed. This is called the Saytzeff rule.
Note:
You may get confused between unimolecular and bimolecular reactions. Unimolecular reactions are those reactions in which only one reactant molecule participates in the reaction whereas in bimolecular reactions two reactant molecules are involved.
Complete step by step answer:
Let us study all the reactions one by one:
(a) \[{{S}_{N}}1\]

It is non-stereospecific i.e., attacked by nucleophiles which occur from both sides. It has carbocation intermediate and is a unimolecular reaction - rate depends on the concentration of only one reactant.
This reaction gives a racemic mixture.
(b) \[{{S}_{N}}2\]

It is a stereospecific reaction because it shows Walden Inversion of configuration. All bonds join and break at the same time. It is a bimolecular reaction - rate depends on the concentration of both the reactants.
This reaction gives enantiomers.
(c) \[{{E}_{1}}\]

It is a non-stereospecific reaction, as it follows the Saytzeff rule.
It has carbocation as an intermediate.
It is also unimolecular- the rate depends on the concentration of only one reactant.
This reaction gives 2 equal amounts of products.
(d) \[{{E}_{2}}\]

It is a stereospecific reaction because it has Anti and Syn planar geometry. All bonds join and break at the same time. It is also bimolecular- rate depends on the concentration of the reaction.
This reaction is stereoselective- one product is major.
Hence, option (b) and (d) are correct answers.
Additional information:
> \[{{S}_{N}}2\] reactions are always accompanied by inversion of configuration just in the same way as an umbrella turns inside out in a strong wind. This inversion of configuration is called Walden Inversion.
> In \[{{E}_{2}}\], if the halogen is on any carbon atom within the chain, the alkyl halide can undergo dehydrohalogenation in two or more different directions depending upon the number of different types of beta-hydrogen atoms are present. In such cases a more highly substituted product is formed. This is called the Saytzeff rule.
Note:
You may get confused between unimolecular and bimolecular reactions. Unimolecular reactions are those reactions in which only one reactant molecule participates in the reaction whereas in bimolecular reactions two reactant molecules are involved.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




