
Which of the following radicals is the most stable?
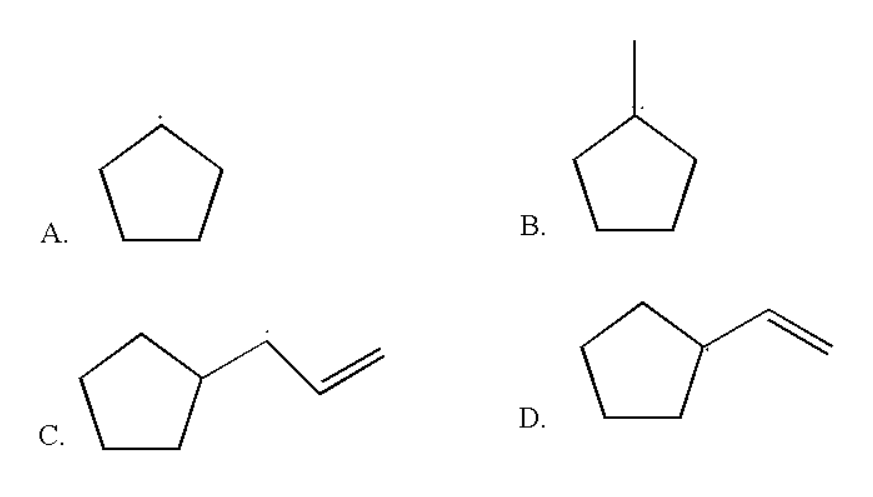

Answer
492.9k+ views
Hint: The stability of radicals depends on the hyperconjugation effect of C-H bonds. The hyperconjugation effect refers to the delocalization of electrons with the single C-H hydrogen. Thus the hyperconjugation effect is mainly due to the sigma bond. The compound which has higher hyperconjugation effect around the free radical will be most stable.
Complete answer:
Radicals are those elements which have unpaired valence electrons. In organic chemistry the radical will be more stable when it is stabilized by certain effects which are resonance effect, methyl substitution, and hyperconjugation effect. The stability of free radicals also follows the same trend as follows by the carbocation. The trend in the stability of the carbocation follows the following trend:
\[{3^ \circ } \succ {2^ \circ } \succ {1^ \circ }\]
Thus three degree free radicals are more stable than two degree free radicals. Also two degree free radicals are more stable than one degree free radicals. Thus from the above given options we can observe the three degree free radical as:
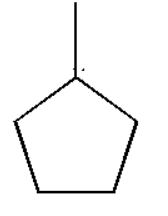
Thus the three degree free radical will be most stable since it has greater hyperconjugation effect than two degree and one degree free radical.
Therefore the correct option is B which is
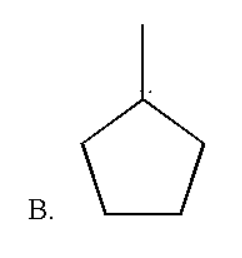
Note:
It must be noted that order of stability of carbocation and free radical is the same while order of stability of carbanion is the reverse. There is no need to verify other options since there is only a single option which is three degree free radical. After the degree of carbon we will check the more hyper conjugated bonds.
Complete answer:
Radicals are those elements which have unpaired valence electrons. In organic chemistry the radical will be more stable when it is stabilized by certain effects which are resonance effect, methyl substitution, and hyperconjugation effect. The stability of free radicals also follows the same trend as follows by the carbocation. The trend in the stability of the carbocation follows the following trend:
\[{3^ \circ } \succ {2^ \circ } \succ {1^ \circ }\]
Thus three degree free radicals are more stable than two degree free radicals. Also two degree free radicals are more stable than one degree free radicals. Thus from the above given options we can observe the three degree free radical as:

Thus the three degree free radical will be most stable since it has greater hyperconjugation effect than two degree and one degree free radical.
Therefore the correct option is B which is

Note:
It must be noted that order of stability of carbocation and free radical is the same while order of stability of carbanion is the reverse. There is no need to verify other options since there is only a single option which is three degree free radical. After the degree of carbon we will check the more hyper conjugated bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




