
Which of the following products is formed when adipic acid is heated?
A.
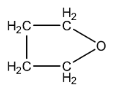
B.
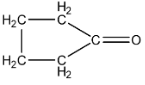
C.
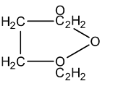
D.





Answer
542.4k+ views
Hint: Adipic acid, on heating loses one carbon dioxide molecule and one water molecule, and cyclization takes place leading to the formation of a ring compound.
Complete step-by-step answer:In order to answer our question, we need to learn about decarboxylation reactions. Decarboxylation is the process by which carbon dioxide molecules are removed from a compound and the carbon dioxide gas is liberated. This can be done by heating, or by using specific reagents or catalysts. Decarboxylation is a reversible process.
When carboxylic acid, containing two carbonyl groups, is heated above ${{150}^{0}}C$, then they lose carbon dioxide atoms readily. However, this reaction is not given by all the carboxylic acids, due to the formation of an unstable carbanion, in cases of other compounds.
Now, let us come to the question. Adipic acid contains two ($-COOH$) groups. This means that on heating, one carbon dioxide molecule and one water molecule can leave. Initially, on heating, the molecule loses one molecule of carbon dioxide, but later on it also loses water. Since charge is deficient, so intermolecular cyclization takes place, and there is formation of a ring structure, with 5 carbon atoms. Hence, the name is given as cyclopentanone.
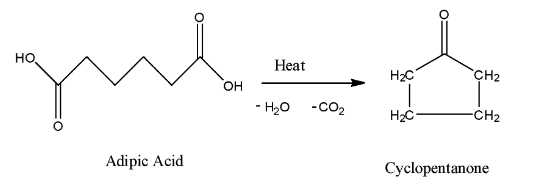
So, we get the correct answer for the question as option B.
Note: It is to be noted that the decarbolised product contains one carbon less than the original reagent. Moreover, decarbonisation of adipic acid is favourable as cyclopentanone is stable due to ring formation.
Complete step-by-step answer:In order to answer our question, we need to learn about decarboxylation reactions. Decarboxylation is the process by which carbon dioxide molecules are removed from a compound and the carbon dioxide gas is liberated. This can be done by heating, or by using specific reagents or catalysts. Decarboxylation is a reversible process.
When carboxylic acid, containing two carbonyl groups, is heated above ${{150}^{0}}C$, then they lose carbon dioxide atoms readily. However, this reaction is not given by all the carboxylic acids, due to the formation of an unstable carbanion, in cases of other compounds.
Now, let us come to the question. Adipic acid contains two ($-COOH$) groups. This means that on heating, one carbon dioxide molecule and one water molecule can leave. Initially, on heating, the molecule loses one molecule of carbon dioxide, but later on it also loses water. Since charge is deficient, so intermolecular cyclization takes place, and there is formation of a ring structure, with 5 carbon atoms. Hence, the name is given as cyclopentanone.

So, we get the correct answer for the question as option B.
Note: It is to be noted that the decarbolised product contains one carbon less than the original reagent. Moreover, decarbonisation of adipic acid is favourable as cyclopentanone is stable due to ring formation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




