
Which of the following oxides of nitrogen is a coloured gas?
A. ${N_2}O$
B.NO
C. ${N_2}{O_4}$
D. $N{O_2}$
Answer
579.9k+ views
Hint: Nitrogen dioxide appears as a reddish-brown gas. $N{O_2}$ along with aerosols, is responsible for the reddish - brown colour of smog. Nitrogen dioxide partners to solid dinitrogen tetroxide at low temperatures.
Complete step by step answer:
Nitrous Oxide $({N_2}O)$ or dinitrogen oxide is also known as Laughing Gas. Nitrous Oxide is a naturally occurring gas that is colourless and sweet-tasting gas. It is a non-flammable gas.

Nitric Oxide (NO) is a colourless gas. It achieves blue shade when it is melted. It is sparingly solvent in water and flammable gas with a slight odour. It is a molecule with a linear shape and is a resonance hybrid of the two structures. The bond length of NO is 115 pm which is intermediate between double bond and triple bond. And it is also paramagnetic in nature.
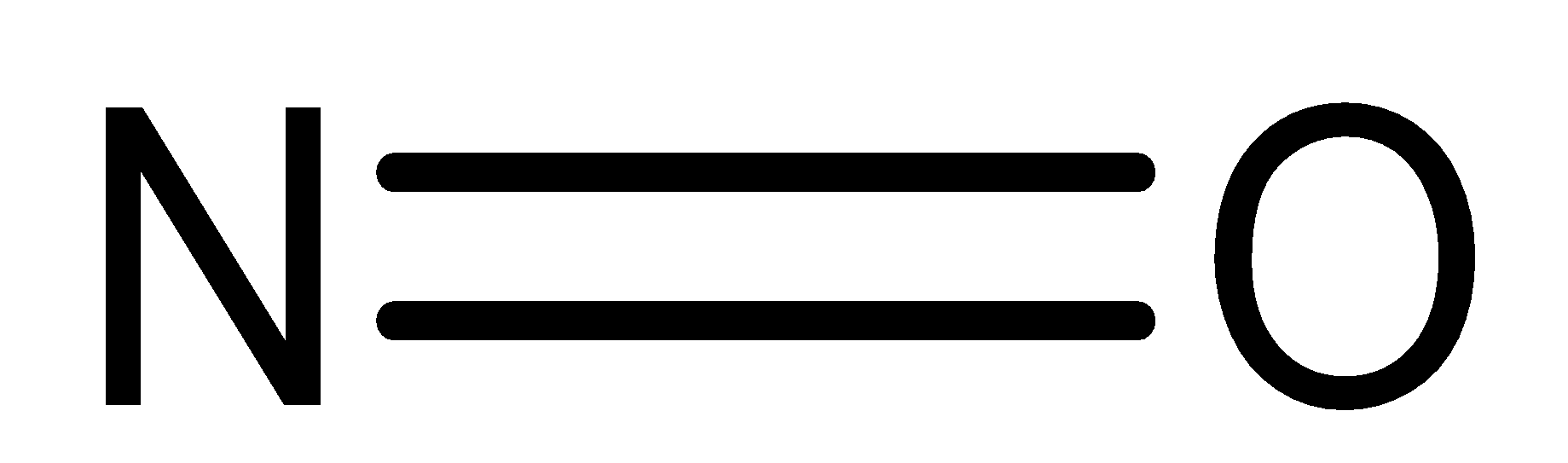
Dinitrogen tetroxide $({N_2}{O_4})$ is a colourless gas. It has an irritating, unpleasant acid-like odour. ${N_2}{O_4}$ is a very reactive, toxic oxidizer. It is non-flammable with air but it will inflame combustible materials.
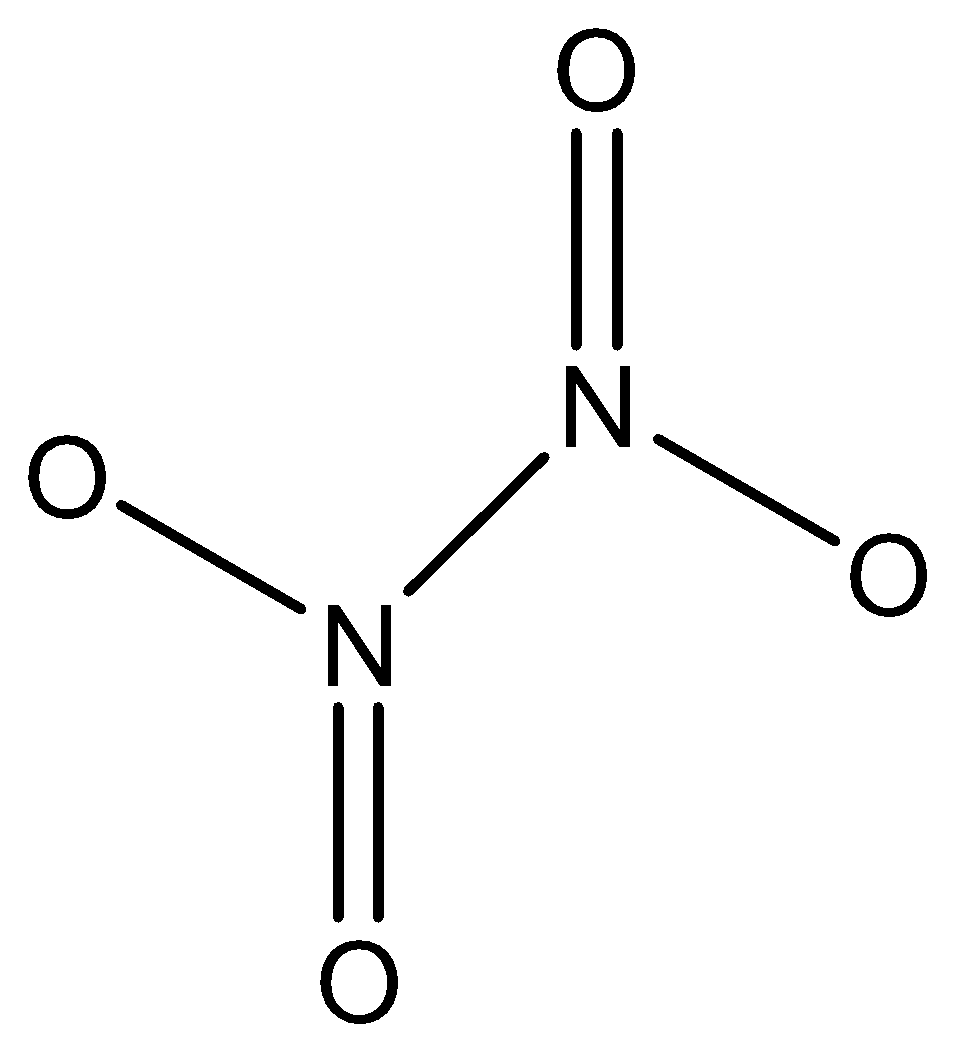
Nitrogen dioxide is a dark reddish-brown gas. It is poisonous but not flammable. $N{O_2}$ along with aerosols, is responsible for the reddish - brown colour of smog. At high concentrations it is highly toxic, and can cause serious lung damage. Nitrogen dioxide is also a strong oxidising agent and thus it is very reactive with other compounds.

Therefore, the correct answer is option (D).
Note: Nitrogen dioxide is an acidic oxide with angular shape. Its bond angle is 134 degrees and bond length is 120 pm. The atom is found as a resonance hybrid and is a paramagnetic substance. Because of the nearness of unpaired electrons, it dimerizes to a colourless dinitrogen tetroxide $({N_2}{O_4})$ atom that has an even number of electrons.
Complete step by step answer:
Nitrous Oxide $({N_2}O)$ or dinitrogen oxide is also known as Laughing Gas. Nitrous Oxide is a naturally occurring gas that is colourless and sweet-tasting gas. It is a non-flammable gas.

Nitric Oxide (NO) is a colourless gas. It achieves blue shade when it is melted. It is sparingly solvent in water and flammable gas with a slight odour. It is a molecule with a linear shape and is a resonance hybrid of the two structures. The bond length of NO is 115 pm which is intermediate between double bond and triple bond. And it is also paramagnetic in nature.
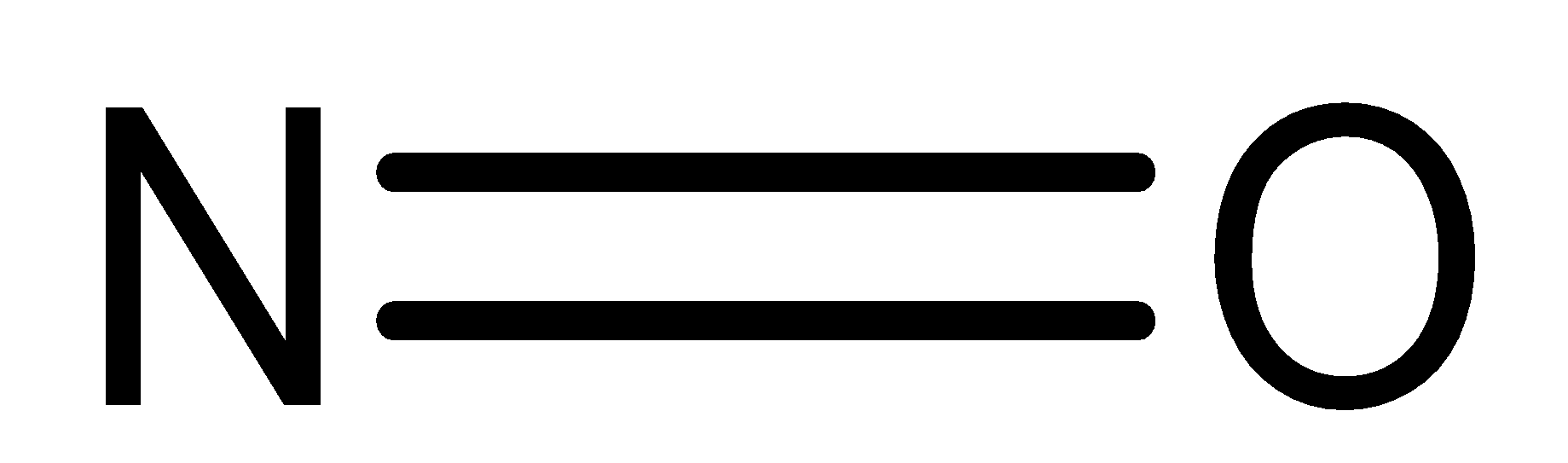
Dinitrogen tetroxide $({N_2}{O_4})$ is a colourless gas. It has an irritating, unpleasant acid-like odour. ${N_2}{O_4}$ is a very reactive, toxic oxidizer. It is non-flammable with air but it will inflame combustible materials.

Nitrogen dioxide is a dark reddish-brown gas. It is poisonous but not flammable. $N{O_2}$ along with aerosols, is responsible for the reddish - brown colour of smog. At high concentrations it is highly toxic, and can cause serious lung damage. Nitrogen dioxide is also a strong oxidising agent and thus it is very reactive with other compounds.

Therefore, the correct answer is option (D).
Note: Nitrogen dioxide is an acidic oxide with angular shape. Its bond angle is 134 degrees and bond length is 120 pm. The atom is found as a resonance hybrid and is a paramagnetic substance. Because of the nearness of unpaired electrons, it dimerizes to a colourless dinitrogen tetroxide $({N_2}{O_4})$ atom that has an even number of electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




