
Which of the following overlapping between p-orbitals would give the strongest bond?
A.
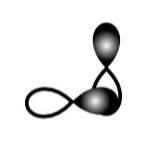
B.
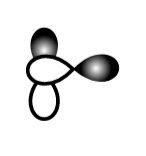
C.
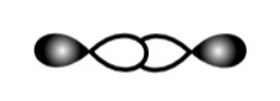
D.
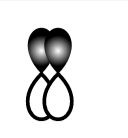




Answer
376.2k+ views
Hint: Overlapping of orbitals has a huge impact on the building of covalent chemical bonds. Partial merging or interpenetration of atomic orbitals is called overlapping which develops in the pairing of electrons.
Complete Step by Step Answer:
Partial merging or interpenetration of atomic orbitals is called overlapping which develops in the pairing of electrons.
The extent of overlap decides the strength of a covalent bond. The greater the overlapping greater will be the strength of the covalent bond.
The covalent bond may be classified into two types depending on the overlapping.
1) sigma bond
This is formed by the end-to-end or head-on overlapping of bonding orbitals along the internuclear axis.
This is known as head-on overlap or axial overlap.
In this case, the overlapping takes place to a larger extent. So, it is a stronger bond than a pi-bond.
2) pi bond
In the formation of the pi bond, the atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis.
The orbitals formed due to side-wise overlapping consist of two saucer-type charge clouds above and below the plane of the participating atoms.
To answer the question, we have to find out which of the following overlapping results in the sigma bond as it is a stronger bond than a pi-bond.
A.
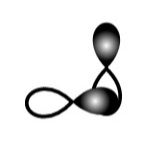
This sidewise overlapping of two p-orbitals so it will form a pi-bond.
So, A is incorrect.
B.
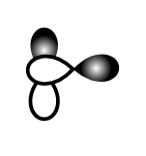
This sidewise overlapping of two p-orbitals so it will form a pi-bond.
So, B is incorrect.
C.
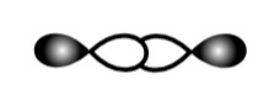
This is head-on p-p overlapping of two half-filled p-orbitals. So, it will form a sigma bond.
So, C is correct.
D.
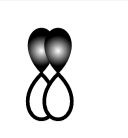
This sidewise overlapping of two p-orbitals so it will form a pi-bond.
So, D is incorrect.
So, option C is correct.
Note: Covalent bond is a chemical bond when the sharing of electron pairs takes place between atoms. The electron pairs which are taken part in the bond formation are shared pairs or bonding pairs.
It must be remarked that in the formation of multiple bonds between two atoms of a molecule, pi bond(s) is created along with a sigma bond.
Complete Step by Step Answer:
Partial merging or interpenetration of atomic orbitals is called overlapping which develops in the pairing of electrons.
The extent of overlap decides the strength of a covalent bond. The greater the overlapping greater will be the strength of the covalent bond.
The covalent bond may be classified into two types depending on the overlapping.
1) sigma bond
This is formed by the end-to-end or head-on overlapping of bonding orbitals along the internuclear axis.
This is known as head-on overlap or axial overlap.
In this case, the overlapping takes place to a larger extent. So, it is a stronger bond than a pi-bond.
2) pi bond
In the formation of the pi bond, the atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis.
The orbitals formed due to side-wise overlapping consist of two saucer-type charge clouds above and below the plane of the participating atoms.
To answer the question, we have to find out which of the following overlapping results in the sigma bond as it is a stronger bond than a pi-bond.
A.

This sidewise overlapping of two p-orbitals so it will form a pi-bond.
So, A is incorrect.
B.

This sidewise overlapping of two p-orbitals so it will form a pi-bond.
So, B is incorrect.
C.

This is head-on p-p overlapping of two half-filled p-orbitals. So, it will form a sigma bond.
So, C is correct.
D.

This sidewise overlapping of two p-orbitals so it will form a pi-bond.
So, D is incorrect.
So, option C is correct.
Note: Covalent bond is a chemical bond when the sharing of electron pairs takes place between atoms. The electron pairs which are taken part in the bond formation are shared pairs or bonding pairs.
It must be remarked that in the formation of multiple bonds between two atoms of a molecule, pi bond(s) is created along with a sigma bond.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Difference Between Plant Cell and Animal Cell




