
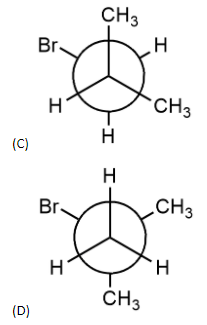
Which of the following Newman’s projections shown below represents the most stable conformation about ${{C}_{1}}-{{C}_{2}}$ bond of 1-Bromo-2-methylpropane?



Answer
583.2k+ views
Hint: Revise the concept of conformations of alkanes. We have studied mainly types of conformers, eclipsed and staggered. In the options, only staggered forms are given and we know staggered conformations are more stable than eclipsed conformation. Now recollect the concept of strain like steric strain and torsional strain and then identify the conformer having the least strain to get the answer.
Complete step by step solution:
- Newman’s projections of alkanes are known as conformations or conformers. They are mainly classified into two types, eclipsed conformations and staggered conformations.
- In the question, only staggered conformations of 1-bromo-2-methylpropane are given. $Br-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{3}}$ is the structure is 1-bromo-2-methylpropane. Here C1 atom is attached as bromine and C2 atom is adjacent to C1 atom.
- Staggered conformations are further divided into two types, anti-staggered and gauche-staggered conformations.
- In anti-staggered conformation, the methyl group and bromine group are at trans position to each other. So, the dihedral angle is $180{}^\circ $. Dihedral angle is the angle between two alkyl groups attached to adjacent carbon atoms forming a bond. An example of this is option (A).
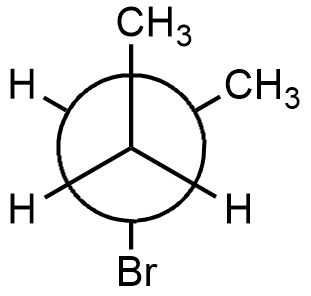
This structure is the most stable structure among all the given options but the structure is of 2-bromobutane and not of 1-bromo-2-methylpropane. In this structure, there is very less steric strain as methyl group and bromine group are trans to each other.
- So, options (A) and (B) can be eliminated since they are conformers of 2-bromobutane.
- Option (D) is also not the structure of 1-bromo-2-methylpropene. It is the structure of 2-bromo-2-methylpropane. Therefore, the answer should be option C.
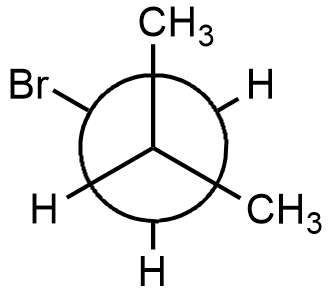
- In option (C), methyl group and bromine group are trans to each other and it is also the correct structure of 1-bromo-2-methylpropane.
- Therefore, the answer is option (C).
Note: Remember to check the structure of the given options with the name of the compound before determining the answer. A simple mistake can lead to confusion and result in wrong answers. Remember anti-form is the most stable conformer of any alkane. Eclipsed conformation is the least stable due to high steric and torsional strain.
Complete step by step solution:
- Newman’s projections of alkanes are known as conformations or conformers. They are mainly classified into two types, eclipsed conformations and staggered conformations.
- In the question, only staggered conformations of 1-bromo-2-methylpropane are given. $Br-C{{H}_{2}}-CH(C{{H}_{3}})-C{{H}_{3}}$ is the structure is 1-bromo-2-methylpropane. Here C1 atom is attached as bromine and C2 atom is adjacent to C1 atom.
- Staggered conformations are further divided into two types, anti-staggered and gauche-staggered conformations.
- In anti-staggered conformation, the methyl group and bromine group are at trans position to each other. So, the dihedral angle is $180{}^\circ $. Dihedral angle is the angle between two alkyl groups attached to adjacent carbon atoms forming a bond. An example of this is option (A).

This structure is the most stable structure among all the given options but the structure is of 2-bromobutane and not of 1-bromo-2-methylpropane. In this structure, there is very less steric strain as methyl group and bromine group are trans to each other.
- So, options (A) and (B) can be eliminated since they are conformers of 2-bromobutane.
- Option (D) is also not the structure of 1-bromo-2-methylpropene. It is the structure of 2-bromo-2-methylpropane. Therefore, the answer should be option C.
- In option (C), methyl group and bromine group are trans to each other and it is also the correct structure of 1-bromo-2-methylpropane.

- Therefore, the answer is option (C).
Note: Remember to check the structure of the given options with the name of the compound before determining the answer. A simple mistake can lead to confusion and result in wrong answers. Remember anti-form is the most stable conformer of any alkane. Eclipsed conformation is the least stable due to high steric and torsional strain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




