
Which of the following molecule(s) has zero dipole moment?
\[
A.{\text{ }}C{H_4} \\
B.{\text{ }}CB{r_4} \\
C.{\text{ }}{C_2}{H_2} \\
D.{\text{ }}none{\text{ }}of{\text{ }}the{\text{ }}above \\
\]
Answer
595.2k+ views
Hint: We must draw the structure of each molecule and then analyse the structure and electronegativity, then only we can determine the dipole moment.
Complete step by step solution:
Let’s start with some basic definition; dipole moment (denoted by Greek letter μ) is the measure of the polarity of chemical bonding between two atoms in a molecule. In the case of electric dipole moment, the direction of the dipole is from electropositive to electronegative. Higher the difference between the electropositive and electronegative higher the value of dipole moment will be. For example, the dipole moment of NaBr will be very high as compared with CBr due to the difference in the electropositive nature of Na and carbon.
So, now we have a basic understanding of what actually a dipole moment is so let’s make the structure and calculate the dipole moment for each.
Case 1:
In case of \[C{H_4}\], even though the shape of methane is tetrahedral but the dipole moment of \[C{H_4}\] will be zero as both the Carbon and hydrogen have minimum polarities as none of them is highly electronegative in nature. \[C{H_4}\] is a symmetrical molecule (µ=0).
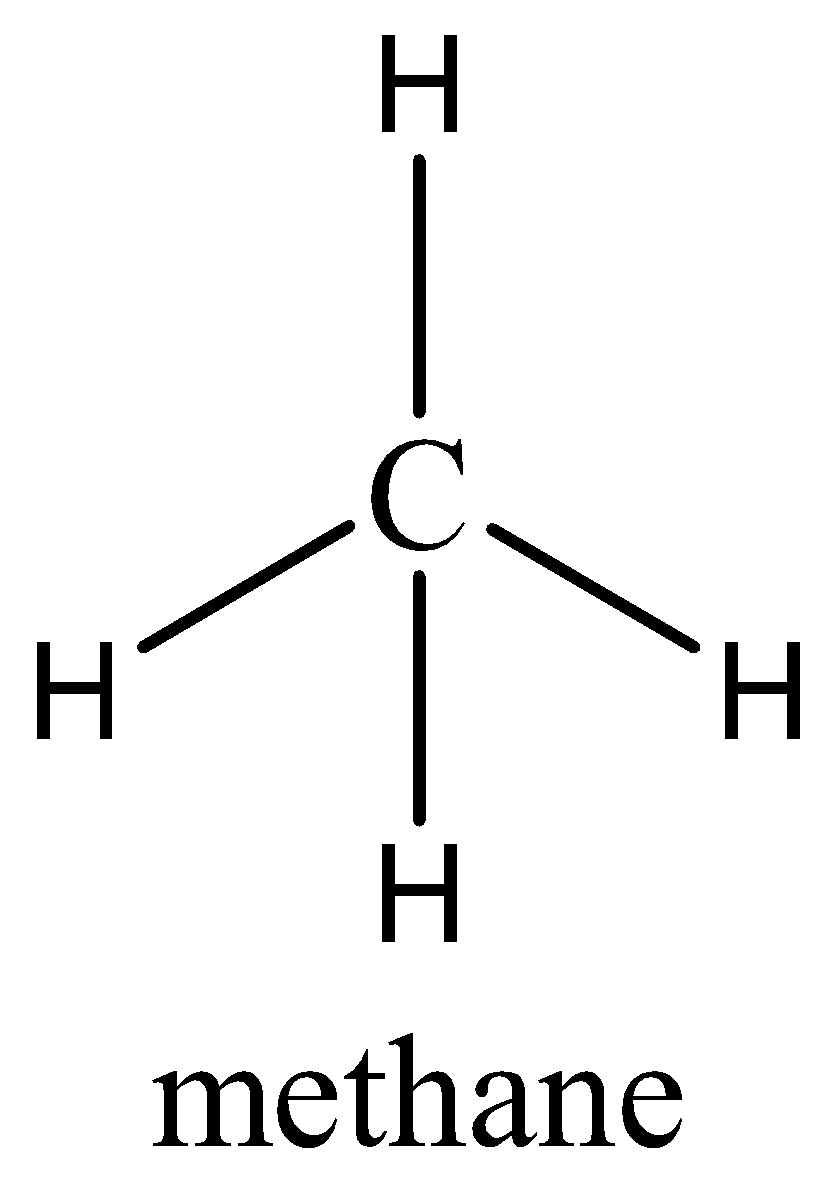
Case 2:
In the case of \[CB{r_4}\], \[Br\] is highly electronegative but due to the tetrahedral structure of \[CB{r_4}\] the individual bond dipoles cancel each other which results in zero dipole moment. And this is a symmetrical molecule (µ=0).
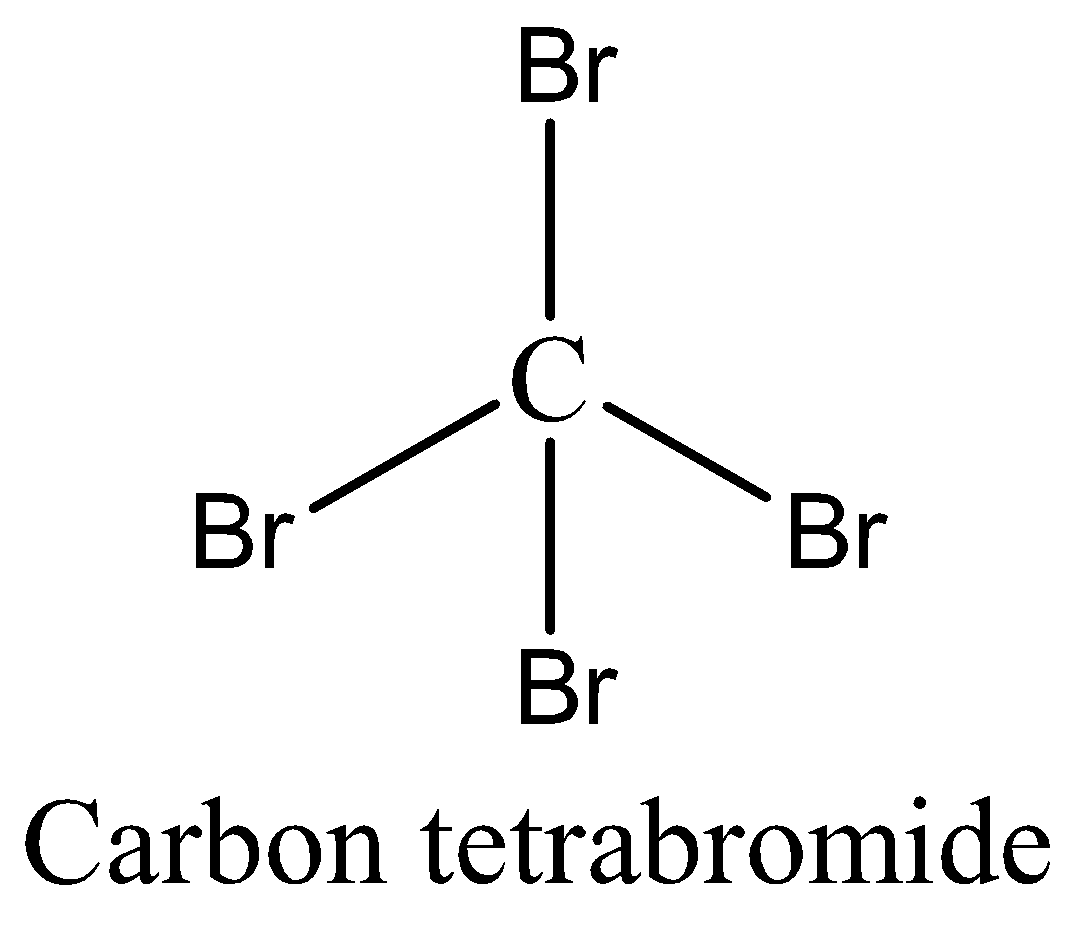
Case 3:
In the case of \[{C_2}{H_2}\], it has a linear structure and it is non-polar in nature along with weak electronegative atoms so it is also having zero dipole moment. And this is a symmetrical molecule (µ=0).
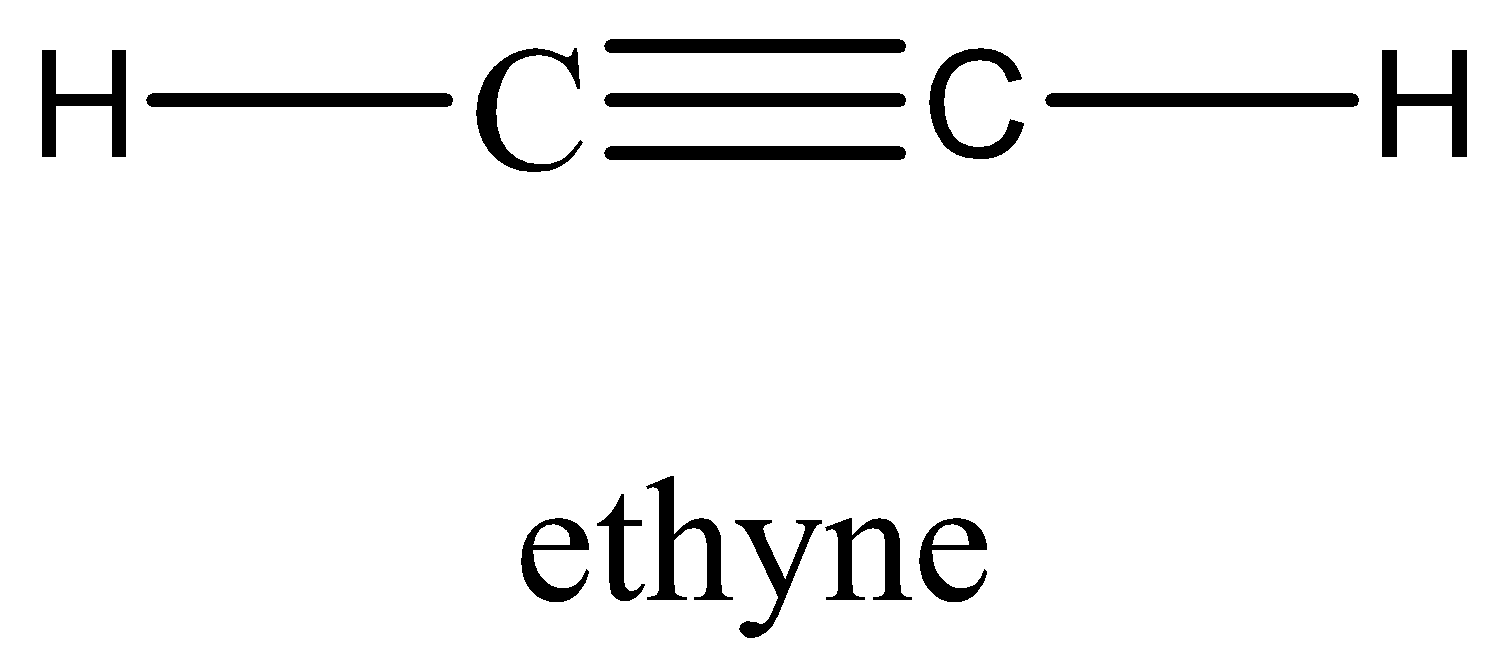
So, we can conclude that the answer to this question will be $C{H_4}{\text{ ,}}CB{r_4}{\text{ ,}}{C_2}{H_2}$.
Note: We must understand that quicklime $CaO$ is used in daily household works and with the demand increasing in its production; industrial revolution is taking place with proper enumeration using it.
Complete step by step solution:
Let’s start with some basic definition; dipole moment (denoted by Greek letter μ) is the measure of the polarity of chemical bonding between two atoms in a molecule. In the case of electric dipole moment, the direction of the dipole is from electropositive to electronegative. Higher the difference between the electropositive and electronegative higher the value of dipole moment will be. For example, the dipole moment of NaBr will be very high as compared with CBr due to the difference in the electropositive nature of Na and carbon.
So, now we have a basic understanding of what actually a dipole moment is so let’s make the structure and calculate the dipole moment for each.
Case 1:
In case of \[C{H_4}\], even though the shape of methane is tetrahedral but the dipole moment of \[C{H_4}\] will be zero as both the Carbon and hydrogen have minimum polarities as none of them is highly electronegative in nature. \[C{H_4}\] is a symmetrical molecule (µ=0).

Case 2:
In the case of \[CB{r_4}\], \[Br\] is highly electronegative but due to the tetrahedral structure of \[CB{r_4}\] the individual bond dipoles cancel each other which results in zero dipole moment. And this is a symmetrical molecule (µ=0).

Case 3:
In the case of \[{C_2}{H_2}\], it has a linear structure and it is non-polar in nature along with weak electronegative atoms so it is also having zero dipole moment. And this is a symmetrical molecule (µ=0).

So, we can conclude that the answer to this question will be $C{H_4}{\text{ ,}}CB{r_4}{\text{ ,}}{C_2}{H_2}$.
Note: We must understand that quicklime $CaO$ is used in daily household works and with the demand increasing in its production; industrial revolution is taking place with proper enumeration using it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




