
Which of the following molecules has the maximum dipole moment?
(A) $C{O_2}$
(B) $C{H_4}$
(C) $N{H_3}$
(D) $N{F_3}$
Answer
579.9k+ views
Hint: Dipole moment can be defined as the product of the distance between the two atoms and the charge on the atoms. The atoms forming bonds need to have differences in electronegativity in order to generate dipole moment.
Complete answer:
When a covalent bond is formed between the same atoms, the pair of electrons is equally attracted by both of the atoms. When a bond is formed between different atoms, the pair of electrons is not equally attracted by both of the atoms. This leads to polarization.
- Due to this polarization the bond possesses a dipole moment. This dipole moment can be defined as the product of the distance between the two atoms and the charge on the atoms.
- $C{O_2}$ :
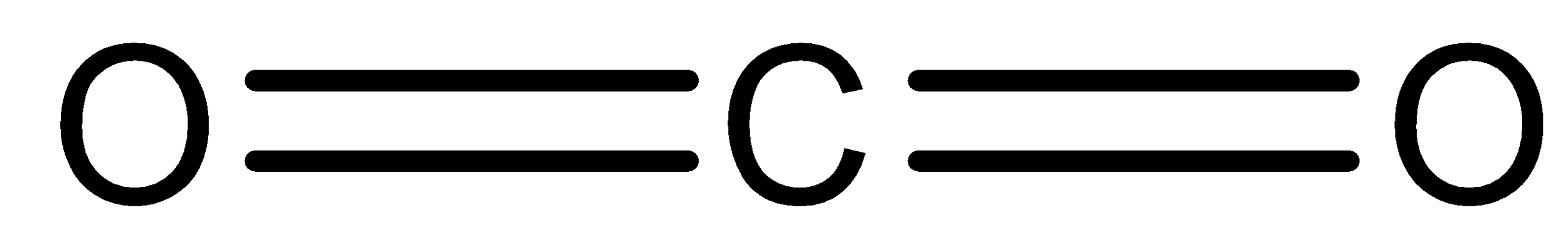
We can see that it is a linear molecule. It involves two C-O double bonds. So, as the electronegativity of carbon atom and oxygen atom is different, there will be some moment in both the bonds. But the direction of the moment will be exactly opposite to each other. So, they will cancel each other. Thus, we can say that it does not have any dipole moment.
- $C{H_4}$ :
This molecule also has a tetrahedral shape. There is a moment present in C-H bond but all the moments will be cancelled as they all nullify their moment upon combination.
- $N{H_3}$ :
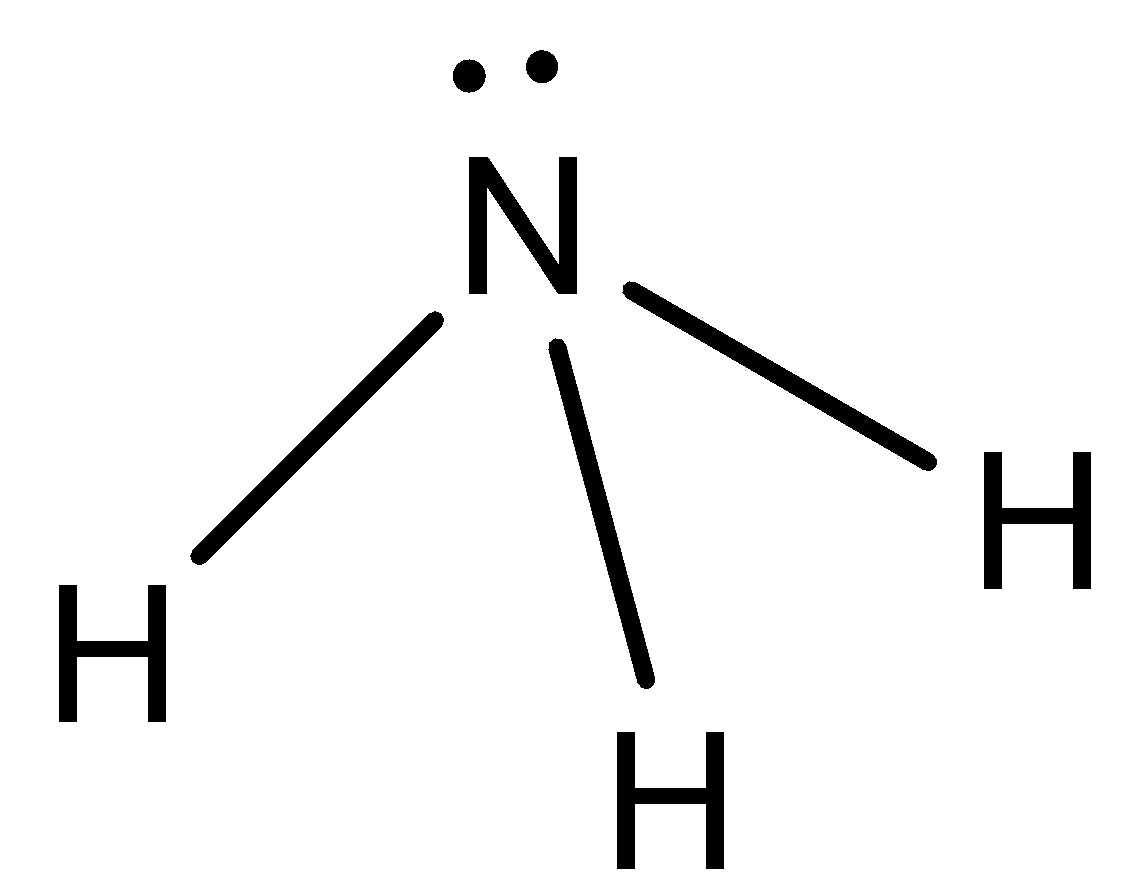
Here, we can see that the difference in electronegativity between N and H atoms is huge. So, there will be some dipole moment in the molecule.
- $N{F_3}$:
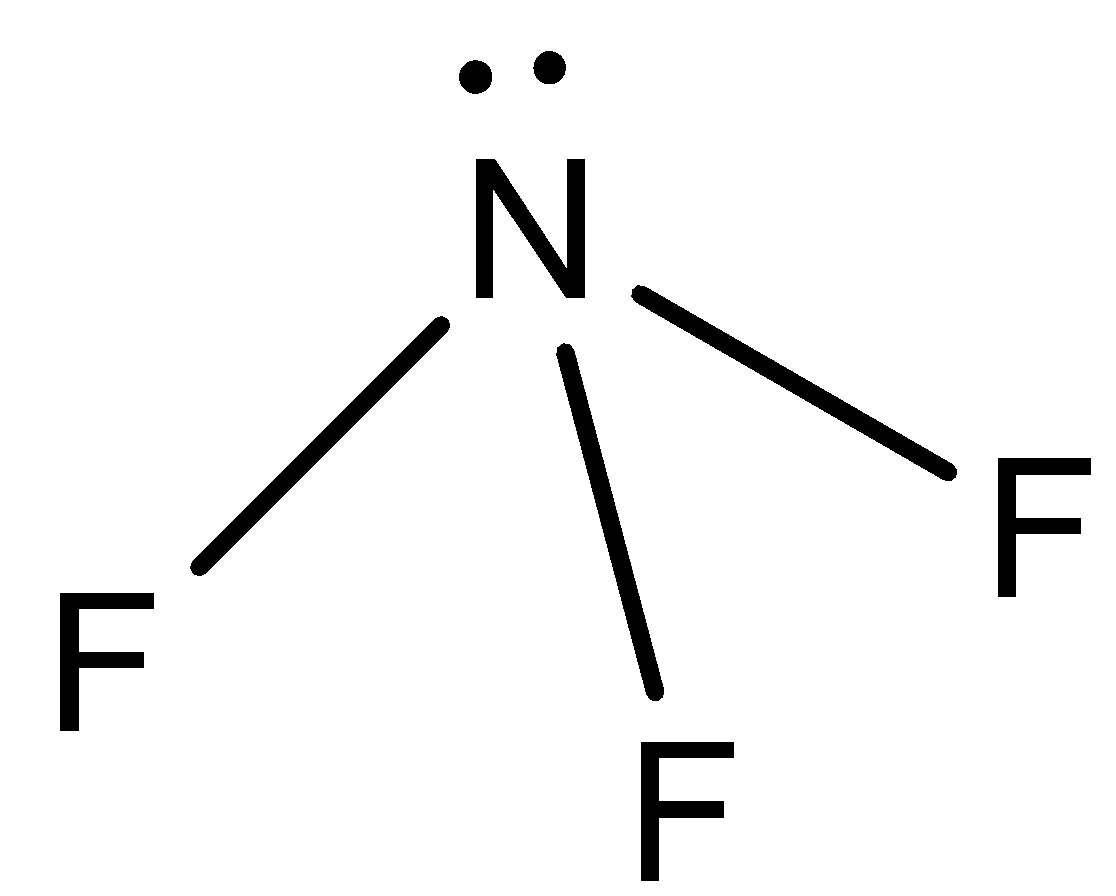
This molecule has the same shape as ammonia but it has fluorine atoms in place of hydrogen atoms. The difference in electronegativity between nitrogen and fluorine atoms is less as compared to N and H atoms. So, there will be less dipole moment in $N{F_3}$ as compared to ammonia.
Thus, we can conclude that the correct answer is (C).
Note:
The dipole moment of a bond is expressed in the Debye unit. It is designated by $\mu $. The resultant dipole moment of $N{H_3}$ is $4.90 \times {10^{ - 30}}Cm$ and that of $N{F_3}$ is $0.80 \times {10^{ - 30}}Cm$.
Complete answer:
When a covalent bond is formed between the same atoms, the pair of electrons is equally attracted by both of the atoms. When a bond is formed between different atoms, the pair of electrons is not equally attracted by both of the atoms. This leads to polarization.
- Due to this polarization the bond possesses a dipole moment. This dipole moment can be defined as the product of the distance between the two atoms and the charge on the atoms.
- $C{O_2}$ :
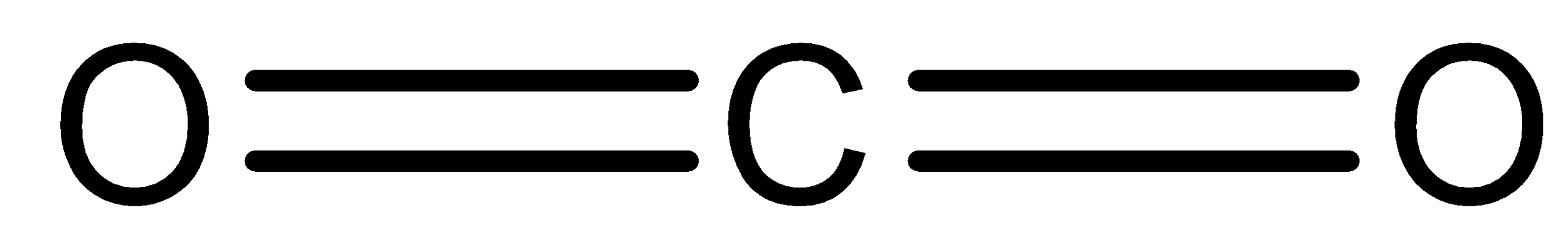
We can see that it is a linear molecule. It involves two C-O double bonds. So, as the electronegativity of carbon atom and oxygen atom is different, there will be some moment in both the bonds. But the direction of the moment will be exactly opposite to each other. So, they will cancel each other. Thus, we can say that it does not have any dipole moment.
- $C{H_4}$ :
This molecule also has a tetrahedral shape. There is a moment present in C-H bond but all the moments will be cancelled as they all nullify their moment upon combination.
- $N{H_3}$ :

Here, we can see that the difference in electronegativity between N and H atoms is huge. So, there will be some dipole moment in the molecule.
- $N{F_3}$:

This molecule has the same shape as ammonia but it has fluorine atoms in place of hydrogen atoms. The difference in electronegativity between nitrogen and fluorine atoms is less as compared to N and H atoms. So, there will be less dipole moment in $N{F_3}$ as compared to ammonia.
Thus, we can conclude that the correct answer is (C).
Note:
The dipole moment of a bond is expressed in the Debye unit. It is designated by $\mu $. The resultant dipole moment of $N{H_3}$ is $4.90 \times {10^{ - 30}}Cm$ and that of $N{F_3}$ is $0.80 \times {10^{ - 30}}Cm$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




