
Which of the following molecules has almost zero dipole moment?
A.
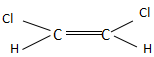
B.
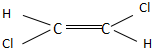
C.
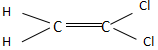
D.
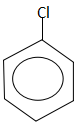
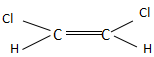
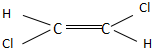
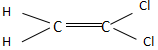

Answer
589.5k+ views
Hint: The dipole moment of the trans compounds are zero as they cancel out each other. In the case of a cis-compound, the dipole moments are in the same direction and thus, it is not considered zero. The compounds which are neither cis nor trans will have a non-zero dipole moment.
Complete step by step answer:
In order to calculate the dipole moment of any compound, we need to find all the dipole moments present in the system and whether they can cancel out each other or not. The direction of dipole moment is always from a less electronegative atom to a more electronegative atom. Let us consider all the four options one by one.
In option A, the dipole moments of both C-Cl bonds is towards upward direction and it is a cis structure. Thus, it does not have a zero dipole moment.
In option B, the dipole moment of one C-Cl bond is towards the left side and the other C-Cl bond is towards the right side. They cancel out each other and produce a stable trans compound. Thus, it will have a zero dipole moment.
In option C, the overall dipole moment is towards the right side and the compound has a non-zero dipole moment.
In option D, the dipole moment is in the upward direction. It does not have a zero dipole moment.
Thus, the correct option is B.
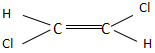 .
.
Note:
In a trans structure, the dipole moments tend to oppose each other because the angle between the two dipole moments is exactly ${180^o}$ . The trans compounds are comparatively more stable than the cis compounds of same molecular formula because the trans compounds have comparatively less energy than the cis compounds.
Complete step by step answer:
In order to calculate the dipole moment of any compound, we need to find all the dipole moments present in the system and whether they can cancel out each other or not. The direction of dipole moment is always from a less electronegative atom to a more electronegative atom. Let us consider all the four options one by one.
In option A, the dipole moments of both C-Cl bonds is towards upward direction and it is a cis structure. Thus, it does not have a zero dipole moment.
In option B, the dipole moment of one C-Cl bond is towards the left side and the other C-Cl bond is towards the right side. They cancel out each other and produce a stable trans compound. Thus, it will have a zero dipole moment.
In option C, the overall dipole moment is towards the right side and the compound has a non-zero dipole moment.
In option D, the dipole moment is in the upward direction. It does not have a zero dipole moment.
Thus, the correct option is B.
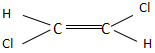
Note:
In a trans structure, the dipole moments tend to oppose each other because the angle between the two dipole moments is exactly ${180^o}$ . The trans compounds are comparatively more stable than the cis compounds of same molecular formula because the trans compounds have comparatively less energy than the cis compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




