
Which of the following isomers of $PB{{r}_{2}}C{{l}_{3}}$ has no dipole moment?
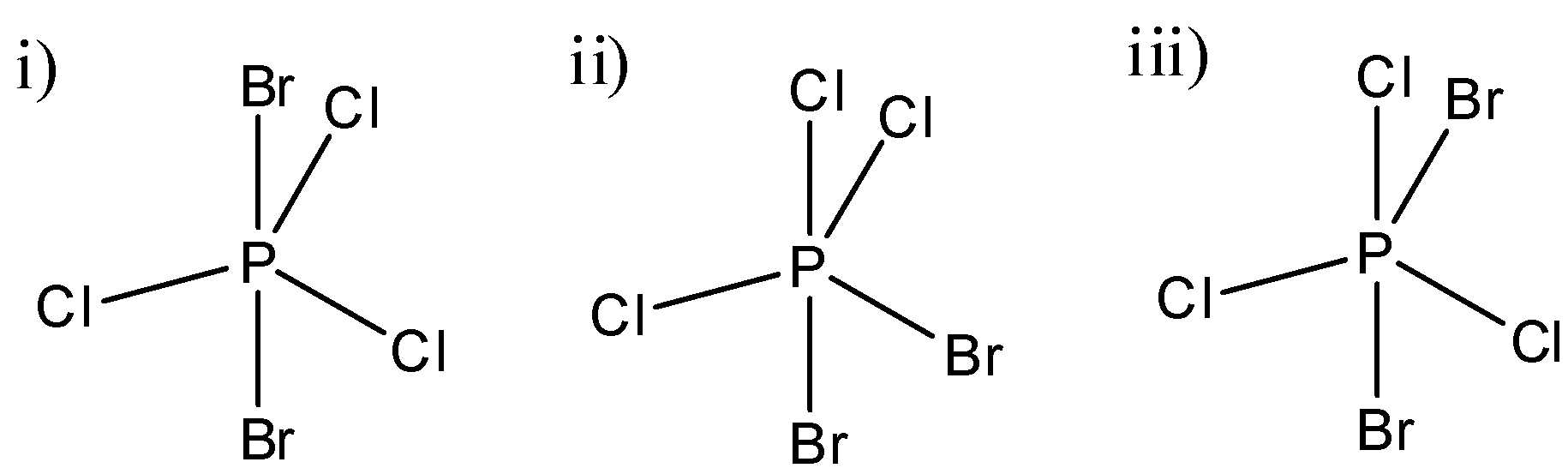
(A) Only I
(B) I and II
(C) II and III
(D) Only III

Answer
595.2k+ views
Hint: Generally, when distribution of dipole is symmetrical, there is no net dipole moment. In order to get an accurate picture of this, we must visualize the shape of the molecule.
Complete answer:
Before trying to solve this question, let us first try and understand what we really mean by the term ‘dipole moment’.
When there is unequal distribution of electrons in the bond, then we can say that the bond has a dipole moment. Dipole moments can be shown by a massive arrow pointing from the partially positive area of the molecule to the partially negative area of the molecule. To illustrate this concept, let us observe the dipole moments of very common molecules.
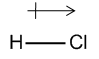
Unfortunately, we can't just calculate dipole moment from Lewis dot structure. Generally, we should take into consideration the shape of the molecule according to the VSEPR theory and the presence of lone pairs, too.
Let’s analyse the structure of given molecules.
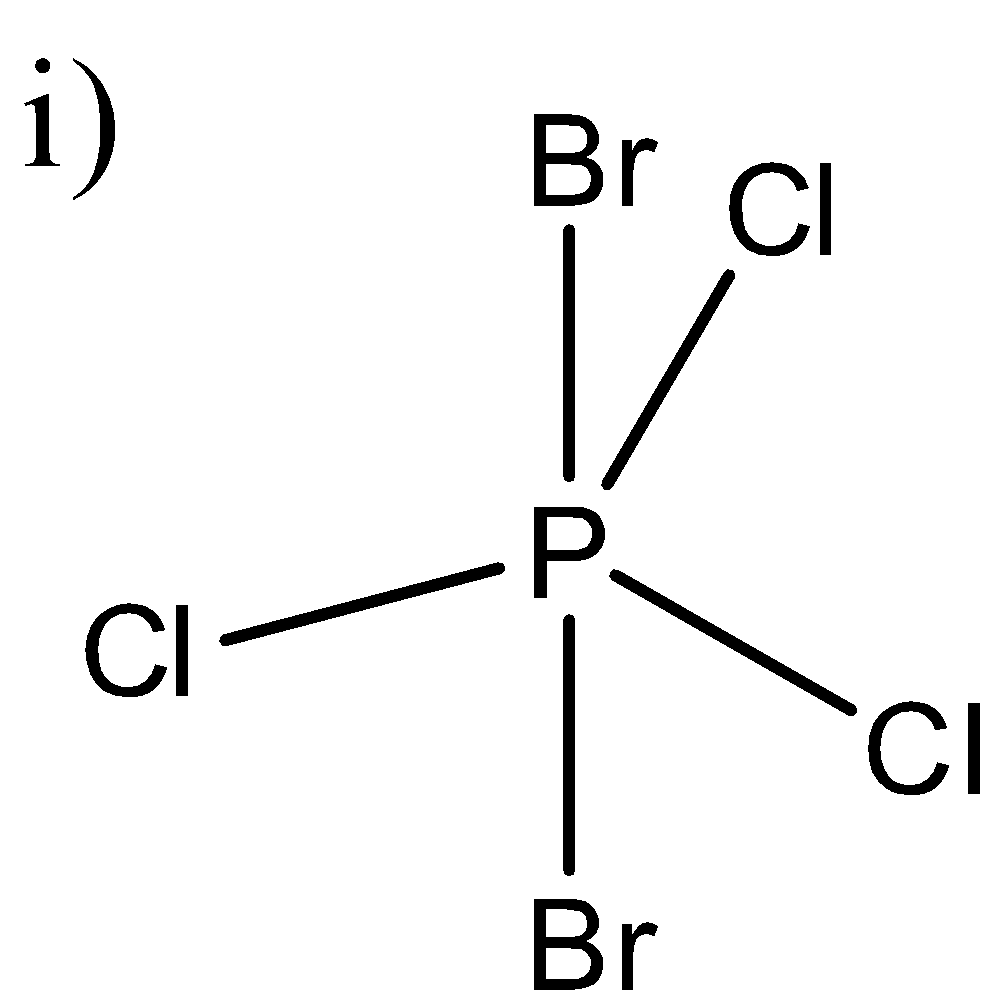
Here, in this molecule, as electronegativity between Br and Cl is different, the dipole moment of P-Br and P-Cl bonds will also be different. Here, the dipole moments of three chlorine atoms will cancel out each other as they are in the same plane. P-Cl bonds will also cancel out each other’s dipole moments as they are at the exact opposite side of the p-atom. So, the resultant dipole moment of this molecule will be equal to zero.
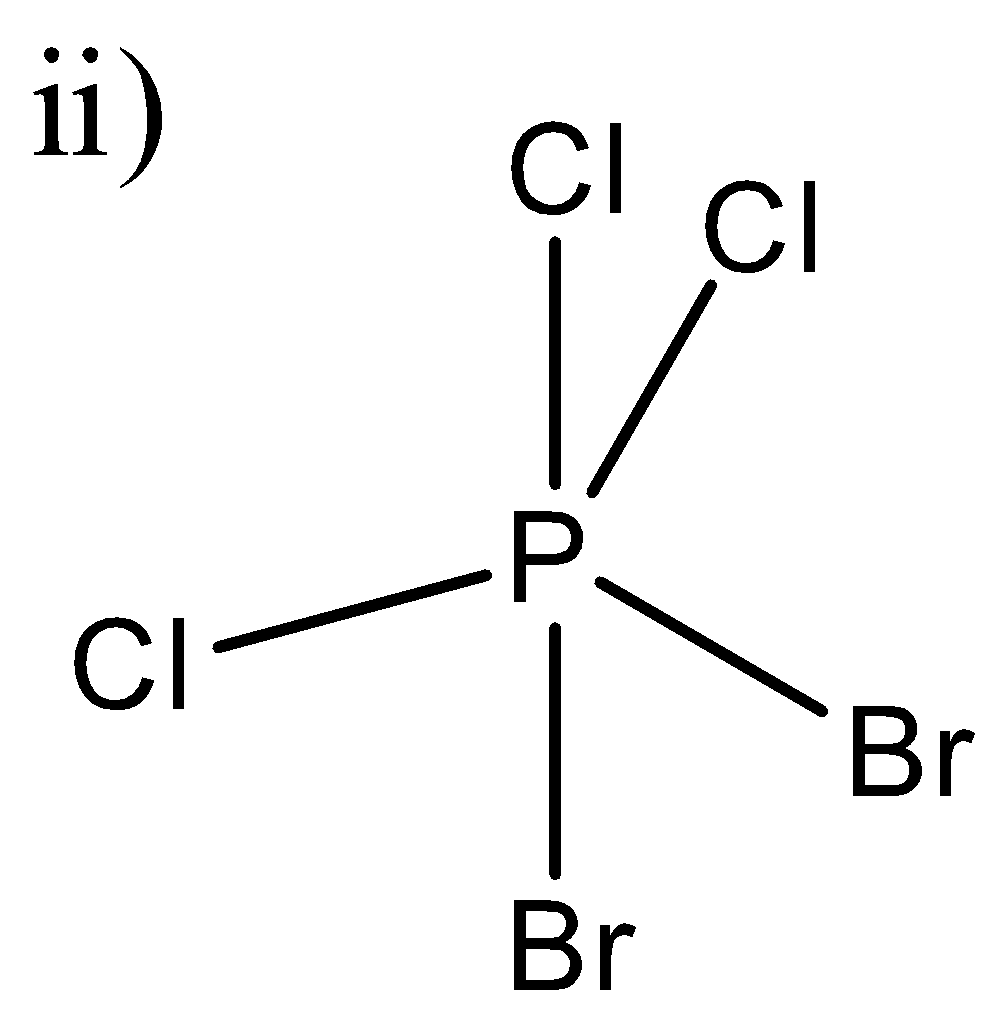
In this molecule, we can see that two chlorine atoms and one bromine atom is in one plane and one Cl-atom and one Br-atom is perpendicular to that plane. So, P-Br and P-Cl dipole moments will be different and hence there will be a resultant dipole moment in this molecule because they will not be able to cancel out each other’s dipole moments. Hence this molecule will have some dipole moment.
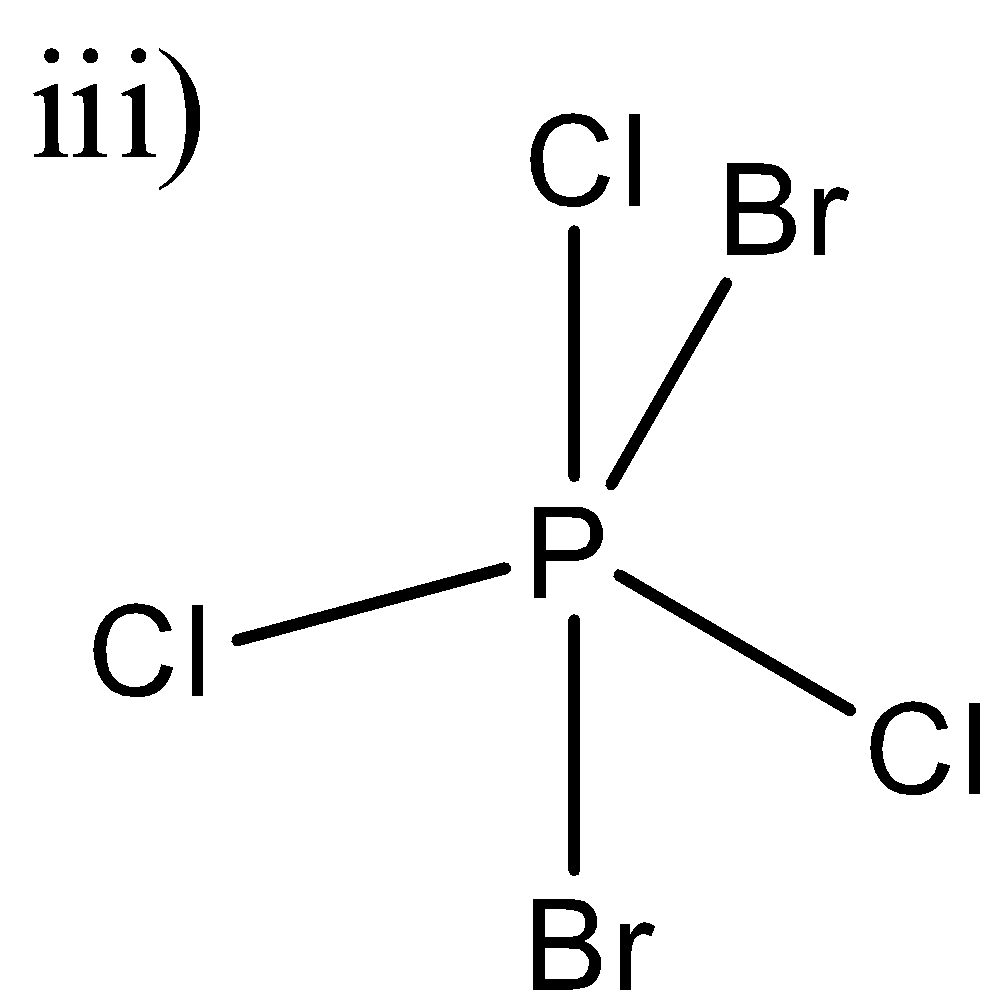
Here, a similar arrangement as the second molecule is there. We can see that in one plane, two chlorine and a bromine atom are there and hence they will not be able to cancel out each other’s effect and as a result this molecule will also have a net dipole moment.
So, we can conclude that only (i) will have zero dipole moment out of these three molecules.
Therefore, the correct answer is (A) Only I.
Note: Remember that dipole moment of a molecule is the net dipole moment of all the bonds. If a compound has zero dipole moment, then it does not mean that compound has only the bonds which do not have dipole moment. As the atoms forming the bond are different, it is evident that dipole moment will be present in minor or major extent, in these cases only if these bonds cancel out each other’s effect, the net dipole moment will be zero.
Complete answer:
Before trying to solve this question, let us first try and understand what we really mean by the term ‘dipole moment’.
When there is unequal distribution of electrons in the bond, then we can say that the bond has a dipole moment. Dipole moments can be shown by a massive arrow pointing from the partially positive area of the molecule to the partially negative area of the molecule. To illustrate this concept, let us observe the dipole moments of very common molecules.

Unfortunately, we can't just calculate dipole moment from Lewis dot structure. Generally, we should take into consideration the shape of the molecule according to the VSEPR theory and the presence of lone pairs, too.
Let’s analyse the structure of given molecules.

Here, in this molecule, as electronegativity between Br and Cl is different, the dipole moment of P-Br and P-Cl bonds will also be different. Here, the dipole moments of three chlorine atoms will cancel out each other as they are in the same plane. P-Cl bonds will also cancel out each other’s dipole moments as they are at the exact opposite side of the p-atom. So, the resultant dipole moment of this molecule will be equal to zero.

In this molecule, we can see that two chlorine atoms and one bromine atom is in one plane and one Cl-atom and one Br-atom is perpendicular to that plane. So, P-Br and P-Cl dipole moments will be different and hence there will be a resultant dipole moment in this molecule because they will not be able to cancel out each other’s dipole moments. Hence this molecule will have some dipole moment.

Here, a similar arrangement as the second molecule is there. We can see that in one plane, two chlorine and a bromine atom are there and hence they will not be able to cancel out each other’s effect and as a result this molecule will also have a net dipole moment.
So, we can conclude that only (i) will have zero dipole moment out of these three molecules.
Therefore, the correct answer is (A) Only I.
Note: Remember that dipole moment of a molecule is the net dipole moment of all the bonds. If a compound has zero dipole moment, then it does not mean that compound has only the bonds which do not have dipole moment. As the atoms forming the bond are different, it is evident that dipole moment will be present in minor or major extent, in these cases only if these bonds cancel out each other’s effect, the net dipole moment will be zero.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




