
Which of the following is/are true about uses of benzoic acid?
(A) Benzoic acid is used as urinary antiseptic.
(B) Salts of benzoic acid are used as urinary antiseptic.
(C) Esters of benzoic acid are used in perfumery.
(D) All of the above.
Answer
578.4k+ views
Hint: Benzoic acid belongs to the class or organic compounds called benzoic acids. Benzoic acids are the organic compounds which contain benzene rings with at least one carboxylic group. Benzoic acid is an aromatic compound in which one carboxylic group is attached to a benzene ring. Benzoic acid has multiple industrial uses.
Complete step by step answer:
Chemical formula of benzoic acid is
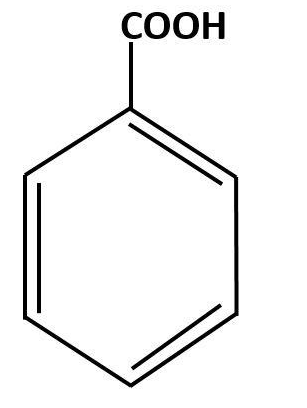
Benzoic acid is the simplest aromatic acid which has only one carboxyl group attached to a benzene ring.
Benzoic acid is a colorless crystalline solid with faint but pleasant odor.
Density of benzoic acid varies with the change in temperature.
Benzoic acid has a melting point of ${122^0}C$
Benzoic acid and some of its salts are used as urinary antiseptics. Therefore, option (A) and (B) are correct.
Sodium salt of benzoic acid is used in food preservatives as it presents strong antibacterial activity at low pH.
Because of its pleasant odor, easter of benzoic acid is used in perfumery. Therefore, option (C) is also correct.
Thus, all above are true about uses of benzoic acid.
Therefore, from the above explanation, the correct answer is, option (D) All of the above.
Note: Even though benzoic acid has its uses in food preservation. But high intake of benzoic acid can be toxic and lead to diarrhea, abdominal pain etc. Therefore, there is a restriction on the amount of benzoic acid that can be used as a food preservative.
Benzoic acid is formed by adding carboxylic acid $( - COOH)$ to benzene and not aldehyde $\left( { - CHO} \right)$. In carboxylic acid, there are two oxygen atoms. While in aldehyde, there is only one oxygen atom.
Complete step by step answer:
Chemical formula of benzoic acid is

Benzoic acid is the simplest aromatic acid which has only one carboxyl group attached to a benzene ring.
Benzoic acid is a colorless crystalline solid with faint but pleasant odor.
Density of benzoic acid varies with the change in temperature.
Benzoic acid has a melting point of ${122^0}C$
Benzoic acid and some of its salts are used as urinary antiseptics. Therefore, option (A) and (B) are correct.
Sodium salt of benzoic acid is used in food preservatives as it presents strong antibacterial activity at low pH.
Because of its pleasant odor, easter of benzoic acid is used in perfumery. Therefore, option (C) is also correct.
Thus, all above are true about uses of benzoic acid.
Therefore, from the above explanation, the correct answer is, option (D) All of the above.
Note: Even though benzoic acid has its uses in food preservation. But high intake of benzoic acid can be toxic and lead to diarrhea, abdominal pain etc. Therefore, there is a restriction on the amount of benzoic acid that can be used as a food preservative.
Benzoic acid is formed by adding carboxylic acid $( - COOH)$ to benzene and not aldehyde $\left( { - CHO} \right)$. In carboxylic acid, there are two oxygen atoms. While in aldehyde, there is only one oxygen atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




