
Which of the following is/are properties of chemisorption?
This question has multiple correct options
(A).Easily liquefied gases are more absorbed
(B). Weak van der waals forces cause the adsorption process
(C). Unimolecular layer formation
(D). Adsorption increases with rise in temperature
Answer
569.4k+ views
Hint:
Chemisorption is the type of adsorption in which adsorbent molecules are attached to adsorbent by the formation of chemical bonds. It is irreversible in nature and also specific. It is favoured by high pressure.
Complete step by step answer:
First of all let us understand about adsorption. Adsorption is the phenomenon of accumulation of molecular species at the surface than in the bulk of a solid or liquid is known as adsorption. The species which gets adsorbed on the surface is called adsorbent while the substance on the surface of which adsorption takes place is called adsorbent. In the case of solid and liquid substances a molecule present in the bulk is equally attracted from all sides and hence forces acting on it are mutually balanced. The molecule present on the surface is attracted only on inner side and hence the are unbalanced or residual attraction force
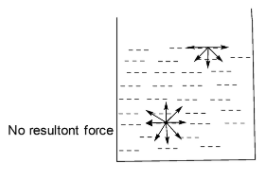
The unbalanced force on the adsorbent surface is responsible for attracting the adsorbent particles on its surface. Thus adsorption is a surface phenomenon.
Depending upon the type of forces that hold the adsorbent on the surface of adsorbent, there are two types of adsorption:
(1). Physical adsorption: When an adsorbent is held to the surface of adsorbent by weak van der waals forces, the adsorption is called physisorption of physical adsorption.
(2). Chemical adsorption: When an adsorbent is held to the surface of adsorbent by chemical bonds, the type of adsorption is called chemisorption. Absorption of hydrogen by metals like $Ni,Pt$ etc. This type of adsorption results in the formation of ‘surface compounds’.
Differences between physisorption and chemisorption properties:
Moreover in chemisorption, easily liquefied gas are more adsorbed and we have discussed that chemisorption increases with increase in temperatures and leads to formation of uni-molecular layers. Hence, option (A), (C) and (D) are the correct answer.
Note: Chemisorption is used in heterogeneous catalysis which involves molecules reacting with each other via the formation of chemisorbed intermediates. Chemisorption is not affected by small changes in the pressure, it is affected by very high pressures.
Chemisorption is the type of adsorption in which adsorbent molecules are attached to adsorbent by the formation of chemical bonds. It is irreversible in nature and also specific. It is favoured by high pressure.
Complete step by step answer:
First of all let us understand about adsorption. Adsorption is the phenomenon of accumulation of molecular species at the surface than in the bulk of a solid or liquid is known as adsorption. The species which gets adsorbed on the surface is called adsorbent while the substance on the surface of which adsorption takes place is called adsorbent. In the case of solid and liquid substances a molecule present in the bulk is equally attracted from all sides and hence forces acting on it are mutually balanced. The molecule present on the surface is attracted only on inner side and hence the are unbalanced or residual attraction force

The unbalanced force on the adsorbent surface is responsible for attracting the adsorbent particles on its surface. Thus adsorption is a surface phenomenon.
Depending upon the type of forces that hold the adsorbent on the surface of adsorbent, there are two types of adsorption:
(1). Physical adsorption: When an adsorbent is held to the surface of adsorbent by weak van der waals forces, the adsorption is called physisorption of physical adsorption.
(2). Chemical adsorption: When an adsorbent is held to the surface of adsorbent by chemical bonds, the type of adsorption is called chemisorption. Absorption of hydrogen by metals like $Ni,Pt$ etc. This type of adsorption results in the formation of ‘surface compounds’.
Differences between physisorption and chemisorption properties:
| Physisorption | Chemisorption |
| (1). The force between adsorbate and weak van der waal forces. | (1). The force between the adsorbate molecule adsorbent are strong chemical forces. |
| (2). It decreases with increase of temperature. | (2). It first increases then decreases with increase of temperature. |
| (3). It results in multi molecular layers on absorbent surfaces under high pressure. | (3). It results in formation of a uni-molecular layer. |
| (4). It does not require any activation energy. | (4). It requires high adsorption energy. |
Moreover in chemisorption, easily liquefied gas are more adsorbed and we have discussed that chemisorption increases with increase in temperatures and leads to formation of uni-molecular layers. Hence, option (A), (C) and (D) are the correct answer.
Note: Chemisorption is used in heterogeneous catalysis which involves molecules reacting with each other via the formation of chemisorbed intermediates. Chemisorption is not affected by small changes in the pressure, it is affected by very high pressures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




