
Which of the following is the strongest base?
a)

b)
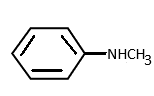
c)

d)
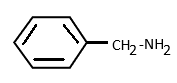




Answer
546.6k+ views
Hint: The basicity depends upon the tendency of a base to donate its lone pair of electrons to accept a proton. This nature can be increased if some electron-containing groups are attached alongside.
Complete step by step solution:
The $ A\min o $ group acts as a strong base as the nitrogen attached has one lone pair of electrons. The electronegativity of nitrogen is also not so high, thus it can donate its lone pair easily.
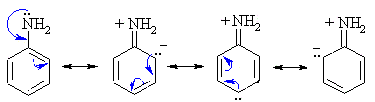
As we know, the benzene ring is an electron rich species with three alternate double bonds. When $ A\min o $ group is attached to a benzene ring, the carbon is $ s{p^2} $ hybridised. Thus as $ s{p^2} $ carbon is more electronegative than $ s{p^3} $ nitrogen, the lone pair of nitrogen are delocalised in a ring having few resonating structures. Hence aniline is not very basic.
N-methyl benzamide is a secondary amine. Here also the lone pair of electrons of nitrogen is delocalised in the ring and thus less available for donation. Hence option (b) it is also not a strong base.
O-toluidine has a methyl group on the ortho position of the amino group. Thus methyl will show +I effect. But the lone pair on nitrogen experiences repulsion from the adjacent hydrogen attached to the methyl group, also known as steric repulsion and will have very less basicity.
In option (d), the carbon attached to nitrogen is $ s{p^3} $ hybridised. Hence as $ s{p^3} $ carbon has very low electronegativity, the lone pair on nitrogen are localised and not attracted to carbon or benzene rings. Thus benzyl amine has the strongest basic nature among all.
$ \therefore $ Option (d) is correct.
Note:
Basicity of a compound depends on the tendency to donate lone pairs of electrons. If the $ {K_b} $ of a base is very high and $ p{K_b} $ value is low ten the base is considered as a strong base.
Complete step by step solution:
The $ A\min o $ group acts as a strong base as the nitrogen attached has one lone pair of electrons. The electronegativity of nitrogen is also not so high, thus it can donate its lone pair easily.
As we know, the benzene ring is an electron rich species with three alternate double bonds. When $ A\min o $ group is attached to a benzene ring, the carbon is $ s{p^2} $ hybridised. Thus as $ s{p^2} $ carbon is more electronegative than $ s{p^3} $ nitrogen, the lone pair of nitrogen are delocalised in a ring having few resonating structures. Hence aniline is not very basic.

N-methyl benzamide is a secondary amine. Here also the lone pair of electrons of nitrogen is delocalised in the ring and thus less available for donation. Hence option (b) it is also not a strong base.
O-toluidine has a methyl group on the ortho position of the amino group. Thus methyl will show +I effect. But the lone pair on nitrogen experiences repulsion from the adjacent hydrogen attached to the methyl group, also known as steric repulsion and will have very less basicity.
In option (d), the carbon attached to nitrogen is $ s{p^3} $ hybridised. Hence as $ s{p^3} $ carbon has very low electronegativity, the lone pair on nitrogen are localised and not attracted to carbon or benzene rings. Thus benzyl amine has the strongest basic nature among all.
$ \therefore $ Option (d) is correct.
Note:
Basicity of a compound depends on the tendency to donate lone pairs of electrons. If the $ {K_b} $ of a base is very high and $ p{K_b} $ value is low ten the base is considered as a strong base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




