
Which of the following is the most substrate most reactive towards methoxide ions?
A.$C{H_3} - I$
B.
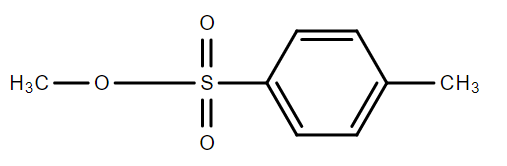
C.$C{H_3} - O - S{O_2} - C{F_3}$
D.$C{H_3} - F$

Answer
574.2k+ views
Hint: To answer this question, you should recall the concept of nucleophilic substitution reactions. The substitution reaction is the type of reaction where a functional group of one chemical compound is substituted by another group or it is a reaction which involves the replacement of one atom or a molecule of a compound with another atom or molecule.
Complete step by step answer:
Methoxide is a strong base hence, will react by the process of $S{N^2}$ or \[{E^2}\]. Since both of these pathways are concerted, each has only one step. Therefore, the rate-limiting transition state given by the rate law is the only transition state of the reaction.
The stereochemistry of each mechanism will be tested by making the \[\alpha \] -carbon a stereocenter. These pathways share a great number of similarities. Both require a good leaving group. $S{N^2}$ reactions require a good nucleophile, while \[{E^2}\] reactions require a good base.
In most cases, however, a good nucleophile is also a good base. Thus $S{N^2}$ and \[{E^2}\] often compete in the same reaction conditions. The winner is determined by the degree of \[\alpha \] and \[\beta \] branching and the strength of the nucleophile/base. Increased \[\alpha \] and \[\beta \] branching and strong basicity favour \[{E^2}\] elimination. Increased nucleophilicity favours the $S{N^2}$ reaction.
Using the above information, we can see that the best option resulting in stable products is option C.
The reaction can be represented as:
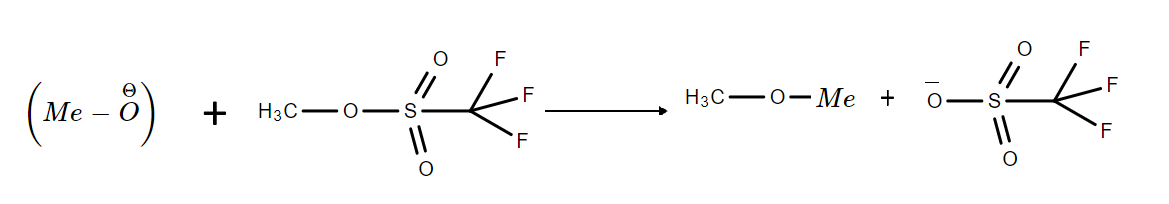
Hence, the correct option is C.
Note:
Make sure you remember the difference between $S{N^1}$ and $S{N^2}$ reaction mechanisms. $S{N^1}$ involves the formation of a carbocation intermediate which is generally in case of tertiary or secondary alkyl halides as their intermediates are stabilised by hyperconjugation with secondary or tertiary alcohols under strongly acidic or strongly basic conditions. The $S{N^1}$ mechanism also is known as a dissociative mechanism. In $S{N^2}$ the reaction mechanism, the nucleophile approaches the given substrate at an angle of\[{180^o}\] to the carbon-leaving group bond. Now, the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre.
Complete step by step answer:
Methoxide is a strong base hence, will react by the process of $S{N^2}$ or \[{E^2}\]. Since both of these pathways are concerted, each has only one step. Therefore, the rate-limiting transition state given by the rate law is the only transition state of the reaction.
The stereochemistry of each mechanism will be tested by making the \[\alpha \] -carbon a stereocenter. These pathways share a great number of similarities. Both require a good leaving group. $S{N^2}$ reactions require a good nucleophile, while \[{E^2}\] reactions require a good base.
In most cases, however, a good nucleophile is also a good base. Thus $S{N^2}$ and \[{E^2}\] often compete in the same reaction conditions. The winner is determined by the degree of \[\alpha \] and \[\beta \] branching and the strength of the nucleophile/base. Increased \[\alpha \] and \[\beta \] branching and strong basicity favour \[{E^2}\] elimination. Increased nucleophilicity favours the $S{N^2}$ reaction.
Using the above information, we can see that the best option resulting in stable products is option C.
The reaction can be represented as:

Hence, the correct option is C.
Note:
Make sure you remember the difference between $S{N^1}$ and $S{N^2}$ reaction mechanisms. $S{N^1}$ involves the formation of a carbocation intermediate which is generally in case of tertiary or secondary alkyl halides as their intermediates are stabilised by hyperconjugation with secondary or tertiary alcohols under strongly acidic or strongly basic conditions. The $S{N^1}$ mechanism also is known as a dissociative mechanism. In $S{N^2}$ the reaction mechanism, the nucleophile approaches the given substrate at an angle of\[{180^o}\] to the carbon-leaving group bond. Now, the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




