
Which of the following is the most stable conformation of cyclohexane?
A. Boat
B. Planar
C. Twist boat
D. Chair
Answer
584.7k+ views
Hint: Conformational isomers is a type of stereoisomerism in which the conformations are interconverted by rotation about $C - C$ sigma bonds and the most stable conformation here is referred to the one which has the lowest energy.
Complete step by step solution:
Firstly, we will learn that why cyclohexane shows conformational isomerism and then about the stabilities of the given conformations,
A regular planar shape of a hexagon has internal angles of ${120^o}$ . However, the carbon-carbon bonds belonging to the cyclohexane ring have a tetrahedral symmetry, with the bond angles almost nearly to ${109.5^o}$ . This is the reason why the cyclohexane ring has a tendency to exhibit conformational isomerism and exhibits a puckered structure to release the strain experienced.
The conformations of cyclohexane known to us are boat, twist-boat, chair and half-chair conformations, and they are named according to the shape that the cyclohexane molecule exhibits.
A. Now, the Boat confirmation is not the most stable conformation because the flagpole hydrogens in the boat conformers experience a repulsion and also experience a torsional stress as nearly all the bonds are eclipsed. Thus, leading to an increase in the energy of the system. Boat confirmation looks like as follows:
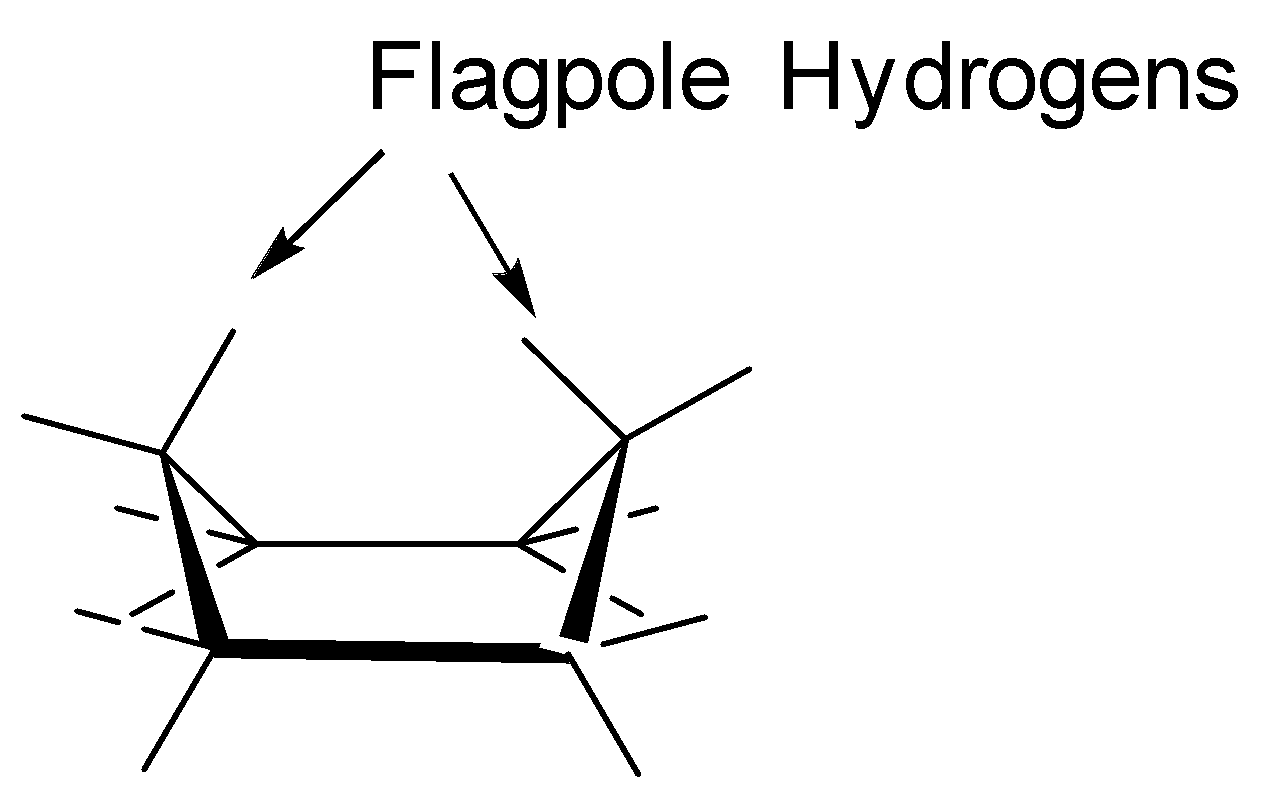
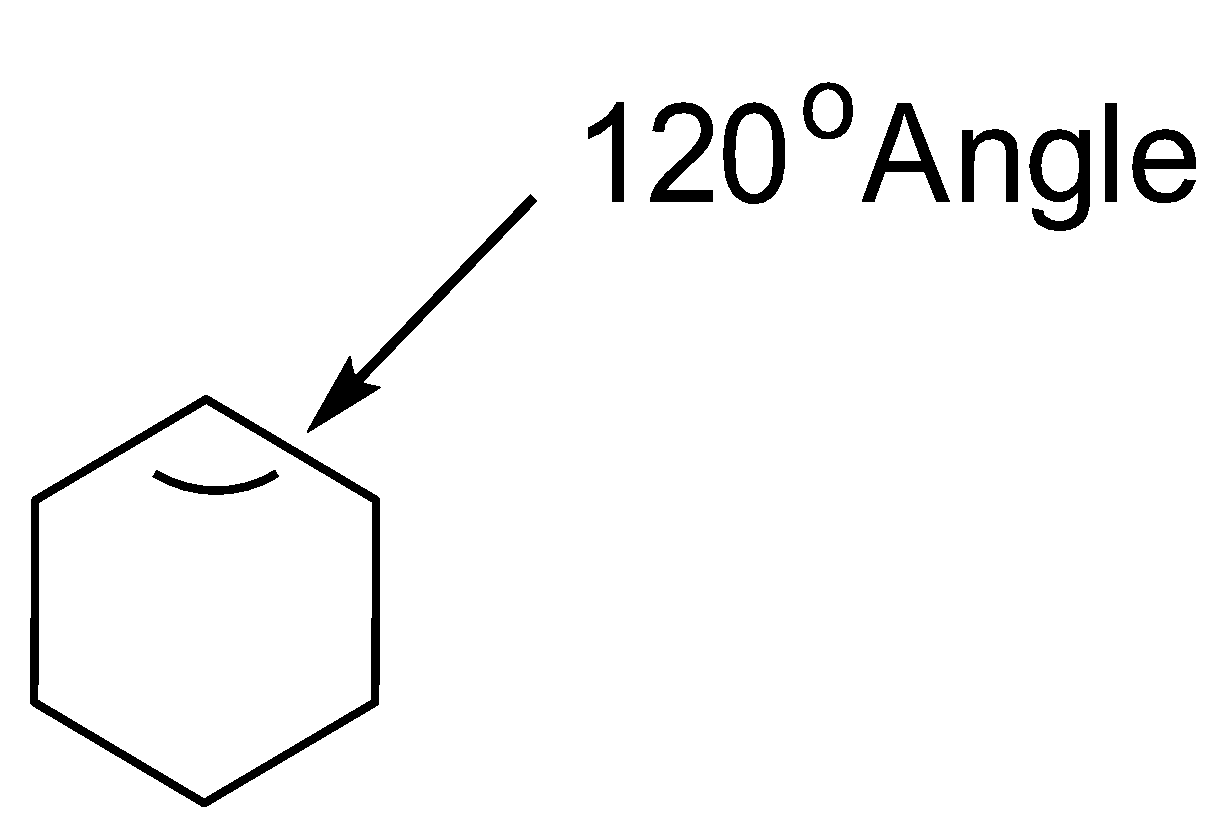
B. The Planar conformation is not the most stable conformation as it experiences a lot of angular strain and thus, showing conformational isomerism so that the angular strain is reduced. The planar conformation looks like as follows:
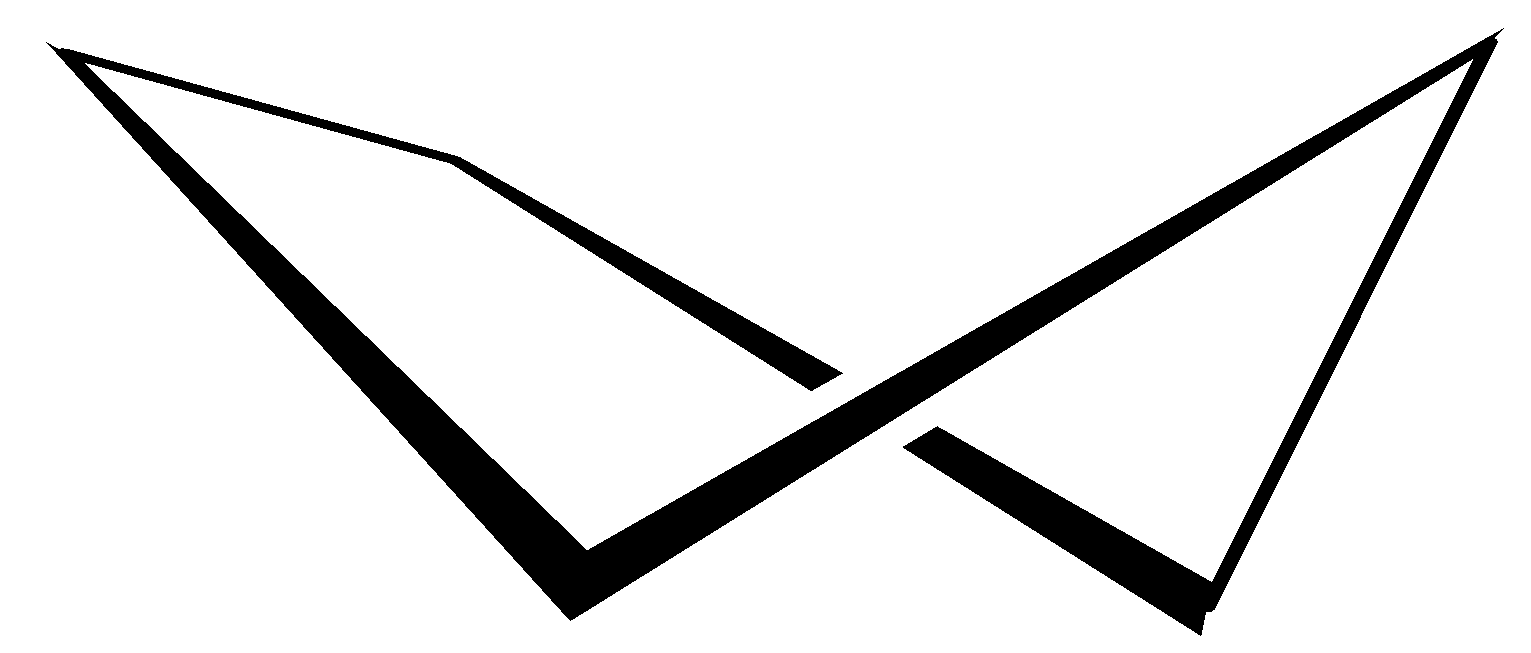
C. The Twist boat conformation is not the most stable conformation but is a stable conformation than the boat conformation, as it has a little less strain than the boat conformer. The twist boat conformation looks like as follows:
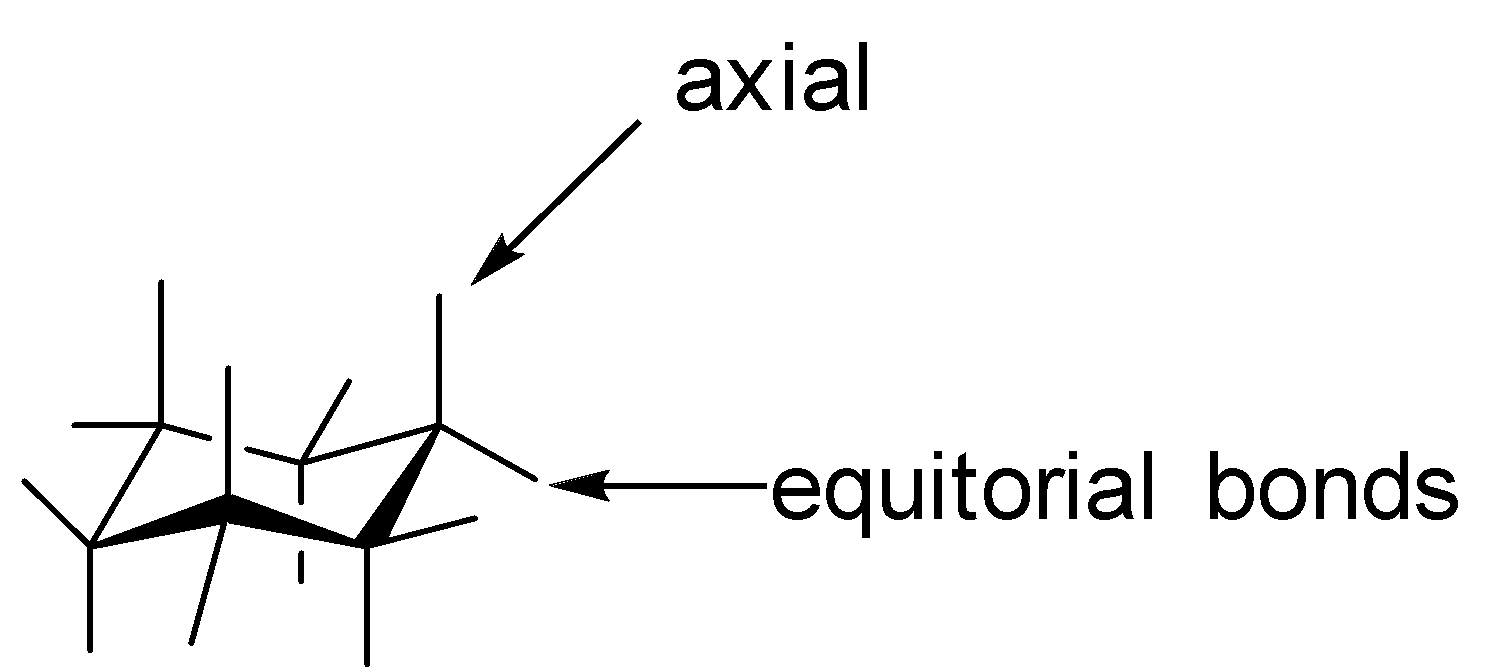
D. The chair form is the most stable conformation of cyclohexane. The $C - C - C$ bonds are very similar to ${109.5^o}$ , so they are almost free from angular strain. And all the bonds in the puckered structure are completely in staggered conformation, and are therefore free of torsional stress. The chair conformation looks like as follows:
So, the most stable conformation of cyclohexane is the chair conformation (Option D).
Note: A student should take care of the fact that all the conformers of cyclohexane are non-planar to release the strain experienced.
Complete step by step solution:
Firstly, we will learn that why cyclohexane shows conformational isomerism and then about the stabilities of the given conformations,
A regular planar shape of a hexagon has internal angles of ${120^o}$ . However, the carbon-carbon bonds belonging to the cyclohexane ring have a tetrahedral symmetry, with the bond angles almost nearly to ${109.5^o}$ . This is the reason why the cyclohexane ring has a tendency to exhibit conformational isomerism and exhibits a puckered structure to release the strain experienced.
The conformations of cyclohexane known to us are boat, twist-boat, chair and half-chair conformations, and they are named according to the shape that the cyclohexane molecule exhibits.
A. Now, the Boat confirmation is not the most stable conformation because the flagpole hydrogens in the boat conformers experience a repulsion and also experience a torsional stress as nearly all the bonds are eclipsed. Thus, leading to an increase in the energy of the system. Boat confirmation looks like as follows:

B. The Planar conformation is not the most stable conformation as it experiences a lot of angular strain and thus, showing conformational isomerism so that the angular strain is reduced. The planar conformation looks like as follows:

C. The Twist boat conformation is not the most stable conformation but is a stable conformation than the boat conformation, as it has a little less strain than the boat conformer. The twist boat conformation looks like as follows:

D. The chair form is the most stable conformation of cyclohexane. The $C - C - C$ bonds are very similar to ${109.5^o}$ , so they are almost free from angular strain. And all the bonds in the puckered structure are completely in staggered conformation, and are therefore free of torsional stress. The chair conformation looks like as follows:

So, the most stable conformation of cyclohexane is the chair conformation (Option D).
Note: A student should take care of the fact that all the conformers of cyclohexane are non-planar to release the strain experienced.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




