
Which of the following is the correct representation when \[B{F_3}\] reacts with ammonia?
1)
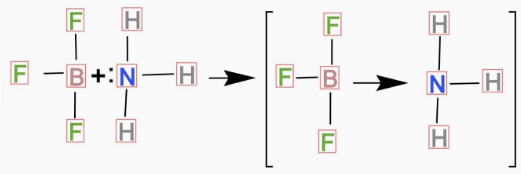
2)
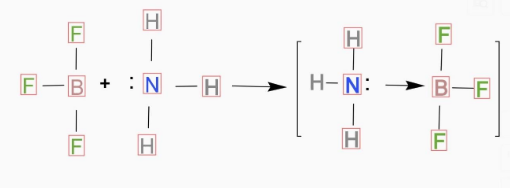
(A) 1 is incorrect and 2 is correct
(B) 1 is correct and 2 is incorrect
(C) Both 1 and 2 are correct
(D) Both 1 and 2 are incorrect


Answer
563.1k+ views
Hint: To answer the question we must know about the concept of Lewis acid and Lewis base. Both \[B{F_3}\]and \[N{H_3}\] are Lewis acid and Lewis base respectively. And we have to know the octet rule and the concept of stable compounds. A detailed discussion is shown below.
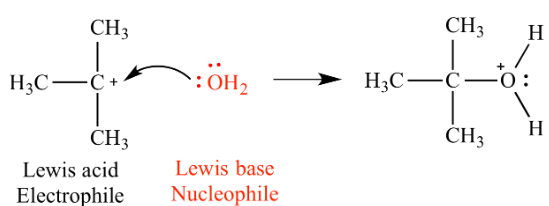
Complete step-by-step answer:Lewis acid: In the Lewis hypothesis of acid-base reactions, bases give sets of electrons and acids acknowledge sets of electrons. A Lewis acid is any substance that can acknowledge a couple of nonbonding electrons. As such, a Lewis acid is an electron-pair acceptor.
One favorable position of the Lewis hypothesis is the manner in which it supplements the model of oxidation-reduction reactions. Oxidation-reduction reactions include an exchange of electrons starting with one particle then onto the next, with a net change in the oxidation number of at least one atom.
Lewis base: A Lewis base is a substance which can donate at least a pair of nonbonding electrons. That means it can be called an electron pair donor.
In the above question, two molecules are \[N{H_3}\]and\[B{F_3}\]. In \[N{H_3}\]there are \[5\] electrons in the outer shell of \[N\] and \[3\]electrons are involved in the bond formation with \[H\] atom and rest of the electrons remains as lone pair that means nonbonding electron-pair. So, we can conclude \[N{H_3}\] is a Lewis base.
And in \[B{F_3}\] there are 3 electrons in the outer shell of \[B\]and all are involved in bond formation so there is no nonbonding electron left in \[B{F_3}\]and we can consider \[B{F_3}\] as a Lewis acid.
So the correct representation for the reaction of \[B{F_3}\] with \[N{H_3}\] will be:

Hence the correct option is (A).
Note:In the reaction it is basically an acid base reaction. When \[B{F_3}\] reacts with \[N{H_3}\] an adduct is formed shown in the above diagram. And in that adduct \[N\] will donate electrons to \[B\] centre.
Complete step-by-step answer:Lewis acid: In the Lewis hypothesis of acid-base reactions, bases give sets of electrons and acids acknowledge sets of electrons. A Lewis acid is any substance that can acknowledge a couple of nonbonding electrons. As such, a Lewis acid is an electron-pair acceptor.
One favorable position of the Lewis hypothesis is the manner in which it supplements the model of oxidation-reduction reactions. Oxidation-reduction reactions include an exchange of electrons starting with one particle then onto the next, with a net change in the oxidation number of at least one atom.
Lewis base: A Lewis base is a substance which can donate at least a pair of nonbonding electrons. That means it can be called an electron pair donor.

In the above question, two molecules are \[N{H_3}\]and\[B{F_3}\]. In \[N{H_3}\]there are \[5\] electrons in the outer shell of \[N\] and \[3\]electrons are involved in the bond formation with \[H\] atom and rest of the electrons remains as lone pair that means nonbonding electron-pair. So, we can conclude \[N{H_3}\] is a Lewis base.
And in \[B{F_3}\] there are 3 electrons in the outer shell of \[B\]and all are involved in bond formation so there is no nonbonding electron left in \[B{F_3}\]and we can consider \[B{F_3}\] as a Lewis acid.
So the correct representation for the reaction of \[B{F_3}\] with \[N{H_3}\] will be:

Hence the correct option is (A).
Note:In the reaction it is basically an acid base reaction. When \[B{F_3}\] reacts with \[N{H_3}\] an adduct is formed shown in the above diagram. And in that adduct \[N\] will donate electrons to \[B\] centre.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




