
Which of the following is not the product of dehydration of:
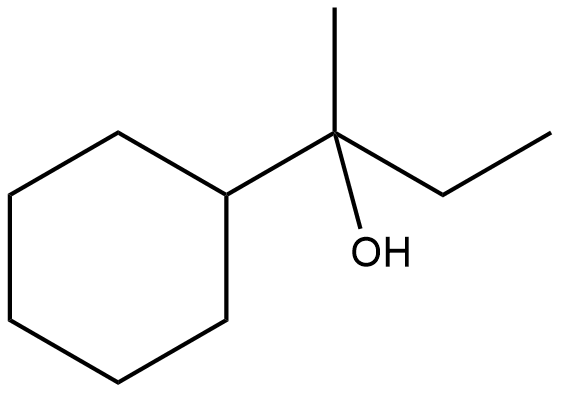
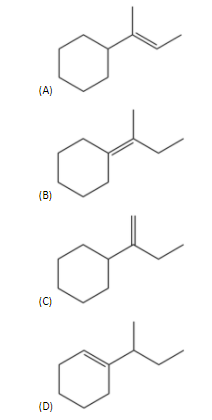


Answer
579.9k+ views
Hint: There is a dehydration reaction taking place for a compound given in the question. Think about what kind of reaction is dehydration and what changes will take place in the compound when dehydration is completely carried out. Try to draw all the possible products in this reaction and then choose the product which is not formed in this reaction as an answer.
Complete step by step solution:
- Dehydration reaction is a process in which water molecules are removed from the reactant to give product.
- Here, the given compound is an alcohol. We know, alcohols undergo dehydration in the presence of a strong dehydrating agent like sulphuric acid or phosphoric acid to form alkenes.
- So, dehydration is an elimination reaction. This reaction is also known as $\text{ }\!\!\alpha\!\!\text { , } \!\!\beta\!\!\text{ -elimination}$ reaction because one hydroxyl (-OH) group which at $\text{ }\!\!\alpha\!\!\text{ -carbon}$ and one hydrogen atom (-H) which is at adjacent $\text{ }\!\!\beta\!\!\text{ -carbon}$ is eliminated in the form of water to give an alkene.
- A general reaction can be represented as,
\[C{{H}_{3}}-C{{H}_{2}}-OH\xrightarrow{{{H}_{2}}S{{O}_{4}}}C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O\]
- Now, let’s write the reaction of the given compound.
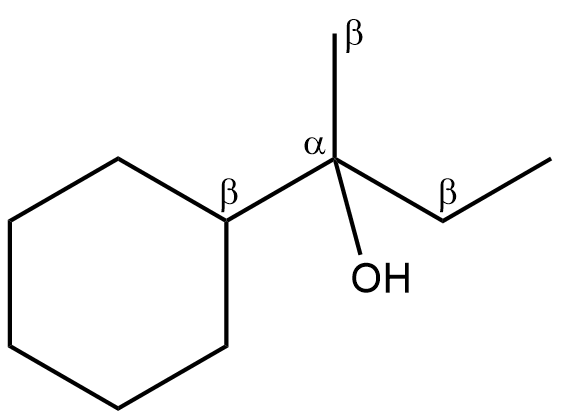
- The double bond will be formed in between the $\text{ }\!\!\alpha\!\!\text{ -carbon}$ and the $\text{ }\!\!\beta\!\!\text{ -carbon}$ atoms which are losing a water molecule. So, the given alcohol molecule has three $\text{ }\!\!\beta\!\!\text{ -hydrogen}$ atoms and therefore, three elimination products are possible.
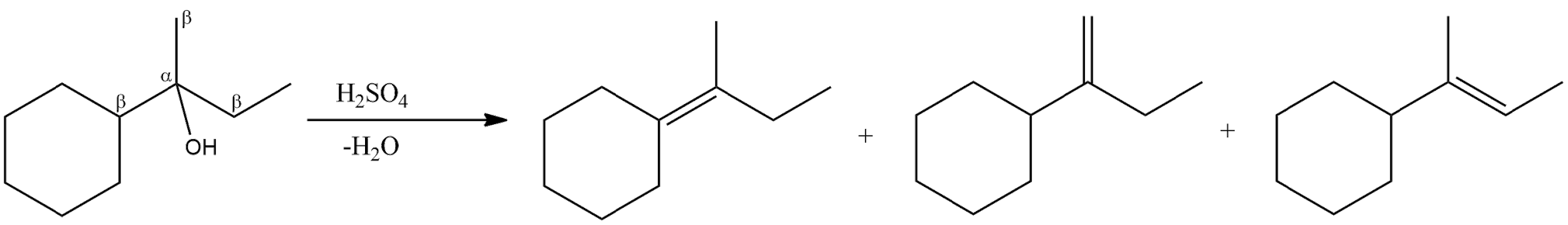
- So, in the options (A), (B) and (C) are the products of the given alcohol compound.
- Therefore, option (D) is the correct answer.
Note: Remember in dehydration reaction, there is elimination of water molecules from the compound. In the case of alcohols and halogen derivatives of alkanes, dehydration reaction will give rise to alkenes as the product by removal of adjacent hydroxyl groups and hydrogen atoms in the form of water. The double bond will be formed in between the $\text{ }\!\!\alpha\!\!\text{ -carbon}$ and the $\text{ }\!\!\beta\!\!\text{ -carbon}$ atoms which are losing a water molecule.
Complete step by step solution:
- Dehydration reaction is a process in which water molecules are removed from the reactant to give product.
- Here, the given compound is an alcohol. We know, alcohols undergo dehydration in the presence of a strong dehydrating agent like sulphuric acid or phosphoric acid to form alkenes.
- So, dehydration is an elimination reaction. This reaction is also known as $\text{ }\!\!\alpha\!\!\text { , } \!\!\beta\!\!\text{ -elimination}$ reaction because one hydroxyl (-OH) group which at $\text{ }\!\!\alpha\!\!\text{ -carbon}$ and one hydrogen atom (-H) which is at adjacent $\text{ }\!\!\beta\!\!\text{ -carbon}$ is eliminated in the form of water to give an alkene.
- A general reaction can be represented as,
\[C{{H}_{3}}-C{{H}_{2}}-OH\xrightarrow{{{H}_{2}}S{{O}_{4}}}C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O\]
- Now, let’s write the reaction of the given compound.

- The double bond will be formed in between the $\text{ }\!\!\alpha\!\!\text{ -carbon}$ and the $\text{ }\!\!\beta\!\!\text{ -carbon}$ atoms which are losing a water molecule. So, the given alcohol molecule has three $\text{ }\!\!\beta\!\!\text{ -hydrogen}$ atoms and therefore, three elimination products are possible.

- So, in the options (A), (B) and (C) are the products of the given alcohol compound.
- Therefore, option (D) is the correct answer.
Note: Remember in dehydration reaction, there is elimination of water molecules from the compound. In the case of alcohols and halogen derivatives of alkanes, dehydration reaction will give rise to alkenes as the product by removal of adjacent hydroxyl groups and hydrogen atoms in the form of water. The double bond will be formed in between the $\text{ }\!\!\alpha\!\!\text{ -carbon}$ and the $\text{ }\!\!\beta\!\!\text{ -carbon}$ atoms which are losing a water molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




