
Which of the following is not the correct name for aspirin?
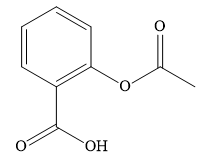
A ) \[{\text{2 - Acetyl salicylic acid}}\]
B ) \[{\text{2 - Acetoxy benzoic acid}}\]
C ) \[{\text{2 - Acetoxy salicylic acid}}\]
D ) None

Answer
583.8k+ views
Hint: Out of two groups \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CO}} - \] and \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CO}} - {\text{O}} - \] , identify which group is acetyl group and which group is acetoxy group.
Salicylic acid is \[{\text{2 - hydroxy benzoic acid}}\] . You can obtain aspirin by acetylation of salicylic acid.
Complete answer:
Aspirin contains a benzene ring, carboxylic group and an acetyl ester of phenol.
Salicylic acid is \[{\text{2 - hydroxy benzoic acid}}\] . When you acetylate salicylic acid, you introduce an acetyl group and obtain aspirin. During acetylation, it acetylates the phenolic hydroxyl group of salicylic acid.
The common name of aspirin is acetyl salicylic acid or \[{\text{2 - acetyl salicylic}}\] acid as an acetyl group is attached to salicylic acid.
The IUPAC name is \[{\text{2 - Acetoxy benzoic acid}}\] acetoxy group is attached to benzoic acid.
Acetyl group is \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CO}} - \] group. Acetoxy group is \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CO}} - {\text{O}} - \] group.
When the acetyl group is attached to salicylic acid, it is the same as the acetoxy group is attached to benzoic acid.
The incorrect name for aspirin is \[{\text{2 - Acetoxy salicylic acid}}\] . Acetoxy groups cannot be attached to salicylic acid. Either acetyl group can be attached to salicylic acid or acetoxy group can be attached to benzoic acid.
Hence, the correct option is C ) \[{\text{2 - Acetoxy salicylic acid}}\].
Note: While naming aromatic compounds containing carboxylic groups directly attached to benzene rings, the parent compound is benzoic acid. Aspirin is derivative of benzoic acid. In aspirin, acetoxy group is present on ortho position (second carbon atom). In esters obtained from acetic acid, acetyl group includes a methyl group and carbonyl group whereas acetoxy group includes acetyl group and an additional oxygen atom.
Salicylic acid is \[{\text{2 - hydroxy benzoic acid}}\] . You can obtain aspirin by acetylation of salicylic acid.
Complete answer:
Aspirin contains a benzene ring, carboxylic group and an acetyl ester of phenol.
Salicylic acid is \[{\text{2 - hydroxy benzoic acid}}\] . When you acetylate salicylic acid, you introduce an acetyl group and obtain aspirin. During acetylation, it acetylates the phenolic hydroxyl group of salicylic acid.
The common name of aspirin is acetyl salicylic acid or \[{\text{2 - acetyl salicylic}}\] acid as an acetyl group is attached to salicylic acid.
The IUPAC name is \[{\text{2 - Acetoxy benzoic acid}}\] acetoxy group is attached to benzoic acid.
Acetyl group is \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CO}} - \] group. Acetoxy group is \[{\text{C}}{{\text{H}}_{\text{3}}} - {\text{CO}} - {\text{O}} - \] group.
When the acetyl group is attached to salicylic acid, it is the same as the acetoxy group is attached to benzoic acid.
The incorrect name for aspirin is \[{\text{2 - Acetoxy salicylic acid}}\] . Acetoxy groups cannot be attached to salicylic acid. Either acetyl group can be attached to salicylic acid or acetoxy group can be attached to benzoic acid.
Hence, the correct option is C ) \[{\text{2 - Acetoxy salicylic acid}}\].
Note: While naming aromatic compounds containing carboxylic groups directly attached to benzene rings, the parent compound is benzoic acid. Aspirin is derivative of benzoic acid. In aspirin, acetoxy group is present on ortho position (second carbon atom). In esters obtained from acetic acid, acetyl group includes a methyl group and carbonyl group whereas acetoxy group includes acetyl group and an additional oxygen atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




