
Which of the following is not expected to be intermediate of the following reaction?
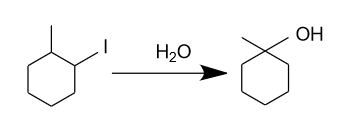
(A)
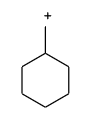
(B)
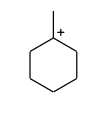
(C)
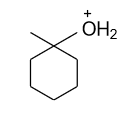
(D)
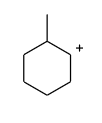





Answer
567k+ views
Hint: Above given reaction is a kind of nucleophilic substitution reaction and iodine is a good leaving group that’s why it easily occurs in the medium of water, where in place of iodine hydroxide ion gets attached.
Complete answer: Above reaction is a nucleophilic substitution reaction which follows the ${\text{S}}{{\text{N}}^{\text{1}}}$ mechanism for giving the final product, through the formation of intermediates.
-Intermediates are those species which are formed during the mechanism from the reactants and then finally convert into the product.
-Above reaction will be completed in multi steps.
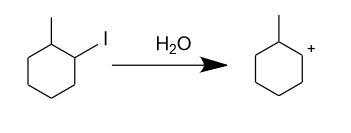
-Firstly in the presence of water iodine removes from the reactant in the form of iodide ion$\left( {{{\text{I}}^{\text{ - }}}} \right)$, and forms the carbocation through the following mechanism.
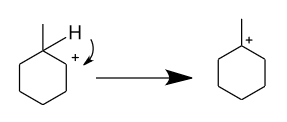
-Now, the formed carbocation do rearrangement to get stability by attaining ${3^0}$ position as at present it is present in the ${2^0}$ position.
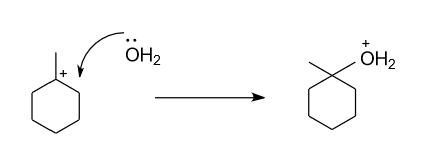
-In the above formed carbocation, water molecule get attack by donating lone pair of electron from oxygen atom of water molecule to the positive charge.
-Previously removed iodide ion in the 1st step now combined with the hydrogen ion, which is removed from the water molecule by balancing the positive charge on the oxygen atom of the water.
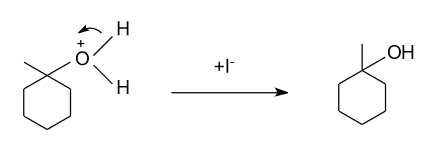
Hence, the intermediate which is given in option (A) is not expected to be intermediate of the given reaction.
Note: Always keep in mind that intermediate is not the reactants that are given in the reaction, it is that species which is formed from the reactant. And the product is formed from the intermediate species.
Complete answer: Above reaction is a nucleophilic substitution reaction which follows the ${\text{S}}{{\text{N}}^{\text{1}}}$ mechanism for giving the final product, through the formation of intermediates.
-Intermediates are those species which are formed during the mechanism from the reactants and then finally convert into the product.
-Above reaction will be completed in multi steps.
-Firstly in the presence of water iodine removes from the reactant in the form of iodide ion$\left( {{{\text{I}}^{\text{ - }}}} \right)$, and forms the carbocation through the following mechanism.

-Now, the formed carbocation do rearrangement to get stability by attaining ${3^0}$ position as at present it is present in the ${2^0}$ position.

-In the above formed carbocation, water molecule get attack by donating lone pair of electron from oxygen atom of water molecule to the positive charge.

-Previously removed iodide ion in the 1st step now combined with the hydrogen ion, which is removed from the water molecule by balancing the positive charge on the oxygen atom of the water.

Hence, the intermediate which is given in option (A) is not expected to be intermediate of the given reaction.
Note: Always keep in mind that intermediate is not the reactants that are given in the reaction, it is that species which is formed from the reactant. And the product is formed from the intermediate species.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




