
Which of the following is not an aromatic compound?
A)

B)
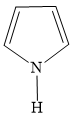
C)
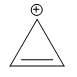
D)
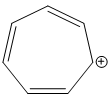




Answer
584.7k+ views
Hint: Follow the Huckel rule of aromaticity. A conjugated, cyclic planar compound having \[\left( {4n + 2} \right){\text{ }}\pi \] electrons is aromatic.
Complete answer:
If the number of pi electrons in the compound is not equal to \[\left( {4n + 2} \right)\] then the compound is non aromatic. For \[n = 0,1,2,3...\] the value of \[\left( {4n + 2} \right)\] is \[4\left( 0 \right) + 2 = 2,4\left( 1 \right) + 2 = 6,{\text{ }}4\left( 2 \right) + 2 = 10,{\text{ }}4\left( 3 \right) + 2 = 14...\] respectively.
Hence, if the number of pi electrons in a compound is not equal to 2,6,10, 14… then it is not an aromatic compound. In the option A ) cycloheptatrienyl anion has 8 pi electrons. Thus the number of pi electrons in cycloheptatrienyl anion is not equal to 2,6,10, 14…
Hence, cycloheptatrienyl anion is not an aromatic compound.
Hence, the correct option is option A ).
Additional Information: The compounds/ions in options B ) and D ) have 6 pi electrons. They are cyclic, planar and conjugated systems of pi electrons. Hence, compounds/ions of options B ) and D ) are aromatic.
The ion of option C ) has 2 pi electrons. It is conjugated, cyclic and planar. Hence, it is aromatic in nature.
Note: While counting the number of pi electrons, each double bond corresponds to a pair of electrons. Each lone pair of electrons corresponds to a pair of electrons. The orbital in which the lone pair of electrons is present, should be parallel to the p orbitals containing pi electrons. If a molecule/ion contains \[\left( {4n + 2} \right){\text{ }}\pi \] pi electrons, but it is non cyclic, non planar or non conjugated, then it is not an aromatic compound.
Complete answer:
If the number of pi electrons in the compound is not equal to \[\left( {4n + 2} \right)\] then the compound is non aromatic. For \[n = 0,1,2,3...\] the value of \[\left( {4n + 2} \right)\] is \[4\left( 0 \right) + 2 = 2,4\left( 1 \right) + 2 = 6,{\text{ }}4\left( 2 \right) + 2 = 10,{\text{ }}4\left( 3 \right) + 2 = 14...\] respectively.
Hence, if the number of pi electrons in a compound is not equal to 2,6,10, 14… then it is not an aromatic compound. In the option A ) cycloheptatrienyl anion has 8 pi electrons. Thus the number of pi electrons in cycloheptatrienyl anion is not equal to 2,6,10, 14…
Hence, cycloheptatrienyl anion is not an aromatic compound.
Hence, the correct option is option A ).
Additional Information: The compounds/ions in options B ) and D ) have 6 pi electrons. They are cyclic, planar and conjugated systems of pi electrons. Hence, compounds/ions of options B ) and D ) are aromatic.
The ion of option C ) has 2 pi electrons. It is conjugated, cyclic and planar. Hence, it is aromatic in nature.
Note: While counting the number of pi electrons, each double bond corresponds to a pair of electrons. Each lone pair of electrons corresponds to a pair of electrons. The orbital in which the lone pair of electrons is present, should be parallel to the p orbitals containing pi electrons. If a molecule/ion contains \[\left( {4n + 2} \right){\text{ }}\pi \] pi electrons, but it is non cyclic, non planar or non conjugated, then it is not an aromatic compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




