
Which of the following is not a nucleophile?
A. $C{{H}_{3}}{{O}^{-}}$
B. ${{H}_{2}}O$
C. $C{{H}_{3}}-OC{{H}_{3}}$
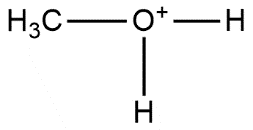
D.

Answer
552.3k+ views
Hint: A nucleophile is generally made up of two words Nucleo and phile where Nucleo refers to nucleus and phile is a Greek word having the meaning love so nucleophiles are also termed as those substances which are nucleus loving.
Complete step by step answer:
- Nucleophiles are generally said to be electron-rich species i.e. those species which have a greater tendency to donate electrons. Due to the nature of donating electrons nucleophiles are kept in the category of Lewis bases as bases are those substances that can donate the \[{{H}^{+}}\] ions. Nucleophiles either carry a negative charge or are neutral in nature.
- The nucleophilic nature of any species describes the affinity of the species towards the positively charged nucleus. All molecules or ions which have a free pair of electrons or at least one pi bond can act as a nucleophile.
- Hence from the above discussion, we can consider that nucleophiles are a chemical species which have the tendency to donate electron pairs to form a chemical bond in a reaction, it can be negative or neutral in charge. From this definition, we can easily conclude that option D is not suitable with a definition of the nucleophile. So, the correct answer is “Option D”.
Note: Nucleophiles generally take part in the nucleophilic substitution reactions and during this reaction nucleophile becomes attracted towards a partial or full positive charge rather than this neutral nucleophilic reaction with solvents like water is known by the name solvolysis.
Complete step by step answer:
- Nucleophiles are generally said to be electron-rich species i.e. those species which have a greater tendency to donate electrons. Due to the nature of donating electrons nucleophiles are kept in the category of Lewis bases as bases are those substances that can donate the \[{{H}^{+}}\] ions. Nucleophiles either carry a negative charge or are neutral in nature.
- The nucleophilic nature of any species describes the affinity of the species towards the positively charged nucleus. All molecules or ions which have a free pair of electrons or at least one pi bond can act as a nucleophile.
- Hence from the above discussion, we can consider that nucleophiles are a chemical species which have the tendency to donate electron pairs to form a chemical bond in a reaction, it can be negative or neutral in charge. From this definition, we can easily conclude that option D is not suitable with a definition of the nucleophile. So, the correct answer is “Option D”.
Note: Nucleophiles generally take part in the nucleophilic substitution reactions and during this reaction nucleophile becomes attracted towards a partial or full positive charge rather than this neutral nucleophilic reaction with solvents like water is known by the name solvolysis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




