
Which of the following is nonpolar?
(A) $S{{O}_{2}}$
(B) $C{{O}_{2}}$
(C) ${{H}_{2}}O$
(D) $N{{H}_{3}}$
Answer
590.1k+ views
Hint: The existence of a hundred percent ionic or covalent bond represents an ideal situation. In reality, no bond or a compound is either completely covalent or ionic. Even in the case of a covalent bond between two hydrogen atoms, there is some ionic character.
Complete step by step answer:
When a covalent bond is formed between two similar atoms, for example in ${{H}_{2}},{{O}_{2}}\ and C{{l}_{2}}$ the shared pair of electrons equally attracted by the two atoms. The bond so formed is called a nonpolar covalent bond.
In the case of heteronuclear molecule HF, the shared electron pair between the two atoms get displaced more towards F is far greater than H. The resultant covalent bond is a polar covalent bond.
As a result of polarization, the molecule possesses the dipole moment, which can be defined as the product of the magnitude of the charge and the distance between the centers of positive and negative charge. It is usually designated by the Greek letter ‘$\mu $ ’and expressed as follows:
Dipole moment ($\mu $) = Charge (Q) x distance of separation (r)
The dipole moment is usually expressed in the Debye unit (D). The conversion factor is
1 D = $3.33564 X {{10}^{-30}}Cm$ , where C is coulomb and m is meter.
In chemistry, the presence of a dipole moment is represented by the crossed arrow () put on the Lewis structure of the molecule. The cross is on the positive end and the arrow head on the negative end.
The dipole moment is related to molecule polarity, which is a vector quantity, and its direction is from electropositive to electronegative element (). The resultant dipole moment is given by the summation of all the vectors.
If the molecule has zero dipole moment and is symmetrical in structure it will be non-polar.
Let’s consider the given molecules,$S{{O}_{2}}$ ,$C{{O}_{2}}$ ,${{H}_{2}}O$ , and $N{{H}_{3}}$ . Out of these four molecules, $C{{O}_{2}}$ is a symmetrical structure. Now check the dipole moment using cross arrow Lewis structure representation,
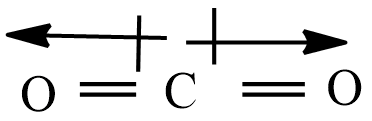
$C{{O}_{2}}$ Is a linear symmetrical structure, hence the summation of all the vectors is zero. So this molecule is nonpolar.
So, the correct answer is “Option B”.
Note: In the case of polyatomic molecules, the dipole moment not only depends upon the individual dipole moments of a bond is known as bond dipoles but also on the spatial arrangement of various bonds in the molecule. Except for $C{{O}_{2}}$, $S{{O}_{2}}$ ,${{H}_{2}}O$, and $N{{H}_{3}}$ are polar covalent bonds.
Complete step by step answer:
When a covalent bond is formed between two similar atoms, for example in ${{H}_{2}},{{O}_{2}}\ and C{{l}_{2}}$ the shared pair of electrons equally attracted by the two atoms. The bond so formed is called a nonpolar covalent bond.
In the case of heteronuclear molecule HF, the shared electron pair between the two atoms get displaced more towards F is far greater than H. The resultant covalent bond is a polar covalent bond.
As a result of polarization, the molecule possesses the dipole moment, which can be defined as the product of the magnitude of the charge and the distance between the centers of positive and negative charge. It is usually designated by the Greek letter ‘$\mu $ ’and expressed as follows:
Dipole moment ($\mu $) = Charge (Q) x distance of separation (r)
The dipole moment is usually expressed in the Debye unit (D). The conversion factor is
1 D = $3.33564 X {{10}^{-30}}Cm$ , where C is coulomb and m is meter.
In chemistry, the presence of a dipole moment is represented by the crossed arrow () put on the Lewis structure of the molecule. The cross is on the positive end and the arrow head on the negative end.
The dipole moment is related to molecule polarity, which is a vector quantity, and its direction is from electropositive to electronegative element (). The resultant dipole moment is given by the summation of all the vectors.
If the molecule has zero dipole moment and is symmetrical in structure it will be non-polar.
Let’s consider the given molecules,$S{{O}_{2}}$ ,$C{{O}_{2}}$ ,${{H}_{2}}O$ , and $N{{H}_{3}}$ . Out of these four molecules, $C{{O}_{2}}$ is a symmetrical structure. Now check the dipole moment using cross arrow Lewis structure representation,
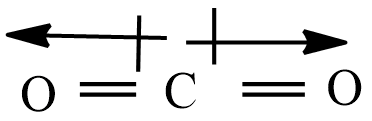
$C{{O}_{2}}$ Is a linear symmetrical structure, hence the summation of all the vectors is zero. So this molecule is nonpolar.
So, the correct answer is “Option B”.
Note: In the case of polyatomic molecules, the dipole moment not only depends upon the individual dipole moments of a bond is known as bond dipoles but also on the spatial arrangement of various bonds in the molecule. Except for $C{{O}_{2}}$, $S{{O}_{2}}$ ,${{H}_{2}}O$, and $N{{H}_{3}}$ are polar covalent bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




