
Which of the following is least likely to behave as Lewis base?
A. $\text{N}{{\text{H}}_{3}}$
B. $\text{B}{{\text{F}}_{3}}$
C. $\text{O}{{\text{H}}^{-}}$
D. ${{\text{H}}_{2}}\text{O}$
Answer
591.6k+ views
Hint: Draw the structures of the structures given, with structure and their configurations. It will be clear that the compound is a Lewis base or Lewis acid. Lewis base is an atomic or molecular species that are electron-rich after their octets are completed and they can donate a pair of electrons while lewis acids have an electron deficient center and they can accept a pair of electrons.
Complete step by step answer:
Let us discuss the options to find out which is less likely to be a Lewis base:
A. $\text{N}{{\text{H}}_{3}}$ : Ammonia has a tetrahedral structure. It is the configuration of 2,5. The valency of nitrogen is 3. Hydrogen atoms have a valency as 1. So, three hydrogen are bonded to nitrogen atoms via a single bond. Thus, the octet of nitrogen is completed. There is one pair of nonbonding electrons present on nitrogen. This lone pair is responsible for its basicity.

B. $\text{B}{{\text{F}}_{3}}$ : Atomic number of boron is 5 and its electronic configuration is 2,3. So, its valency is 3. Fluorine on the other hand has the 2,7 with valency as 1. Thus, sharing one electron with each fluorine atom, boron has 3 sigma bonds with each fluorine atom. Three bonds means there are only 6 electrons around boron. So, in order to have the stable octet configuration, it needs two electrons more. Its planar structure of is:
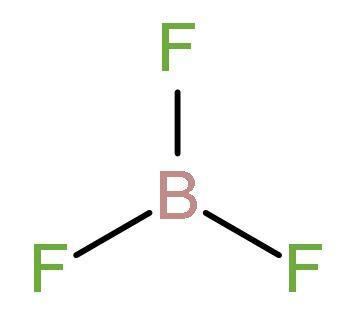
So, it is clear that it is not a Lewis base instead it is a Lewis acid; as it is electron deficient.
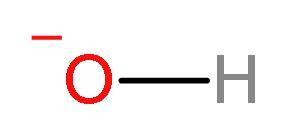
C. $\text{O}{{\text{H}}^{-}}$: $\text{O}{{\text{H}}^{-}}$ion have lone pairs on oxygen atoms and negative charge. So, it can donate a pair of nonbonding electrons. According to the Lewis theory, the base is one which fulfils the electron deficiency of any ion or molecule that can accept a pair of nonbonding valence electrons. $\text{O}{{\text{H}}^{-}}$ is a Lewis base.
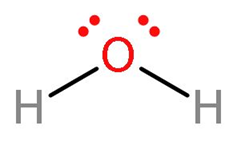
D. ${{\text{H}}_{2}}\text{O}$: Water has bent shape. The configuration of oxygen is 2,6 and valency as 2. So, in water oxygen has formed two sigma bonds with each hydrogen atom. There are two lone pairs present on the water molecule. These lone pairs are the one which water can donate. The structure is:
So, the correct answer is “Option B”.
Note: The terms nucleophiles and Lewis bases are the same. Nucleophiles are the molecules, ions or species with a free pair of electrons or at least one pi bond that can act as nucleophiles. Nucleophiles donate electrons as they are by definition Lewis bases.
Complete step by step answer:
Let us discuss the options to find out which is less likely to be a Lewis base:
A. $\text{N}{{\text{H}}_{3}}$ : Ammonia has a tetrahedral structure. It is the configuration of 2,5. The valency of nitrogen is 3. Hydrogen atoms have a valency as 1. So, three hydrogen are bonded to nitrogen atoms via a single bond. Thus, the octet of nitrogen is completed. There is one pair of nonbonding electrons present on nitrogen. This lone pair is responsible for its basicity.

B. $\text{B}{{\text{F}}_{3}}$ : Atomic number of boron is 5 and its electronic configuration is 2,3. So, its valency is 3. Fluorine on the other hand has the 2,7 with valency as 1. Thus, sharing one electron with each fluorine atom, boron has 3 sigma bonds with each fluorine atom. Three bonds means there are only 6 electrons around boron. So, in order to have the stable octet configuration, it needs two electrons more. Its planar structure of is:

So, it is clear that it is not a Lewis base instead it is a Lewis acid; as it is electron deficient.
C. $\text{O}{{\text{H}}^{-}}$: $\text{O}{{\text{H}}^{-}}$ion have lone pairs on oxygen atoms and negative charge. So, it can donate a pair of nonbonding electrons. According to the Lewis theory, the base is one which fulfils the electron deficiency of any ion or molecule that can accept a pair of nonbonding valence electrons. $\text{O}{{\text{H}}^{-}}$ is a Lewis base.

D. ${{\text{H}}_{2}}\text{O}$: Water has bent shape. The configuration of oxygen is 2,6 and valency as 2. So, in water oxygen has formed two sigma bonds with each hydrogen atom. There are two lone pairs present on the water molecule. These lone pairs are the one which water can donate. The structure is:

So, the correct answer is “Option B”.
Note: The terms nucleophiles and Lewis bases are the same. Nucleophiles are the molecules, ions or species with a free pair of electrons or at least one pi bond that can act as nucleophiles. Nucleophiles donate electrons as they are by definition Lewis bases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




