
Which of the following is known as invert soap?
a.) Pentaerythritol monostearate
b.) Trimethyl stearyl ammonium bromide
c.) Ethoxylated nonylphenol
d.) Sodium stearyl sulphate
Answer
596.1k+ views
Hint: Invert soap is a type of synthetic detergent in which the active part of the surface of the molecule is positively charged (cation). So, cationic detergent is called invert soap. Cation means positively charged ions.
Complete step by step solution:
Invert soap contains a surface which is positively charged, in normal soap a negatively charged surface is the active part.
Coming to the given options, option A, Pentaerythritol monostearate. The molecular formula of
Pentaerythritol monostearate is \[{{C}_{23}}{{H}_{46}}{{O}_{5}}\]. It does not have an active part on the surface of the molecule. So, it is not the correct option.
Coming to option B, Trimethyl stearyl ammonium bromide.
The structure of the Pentaerythritol monostearate is as follows.
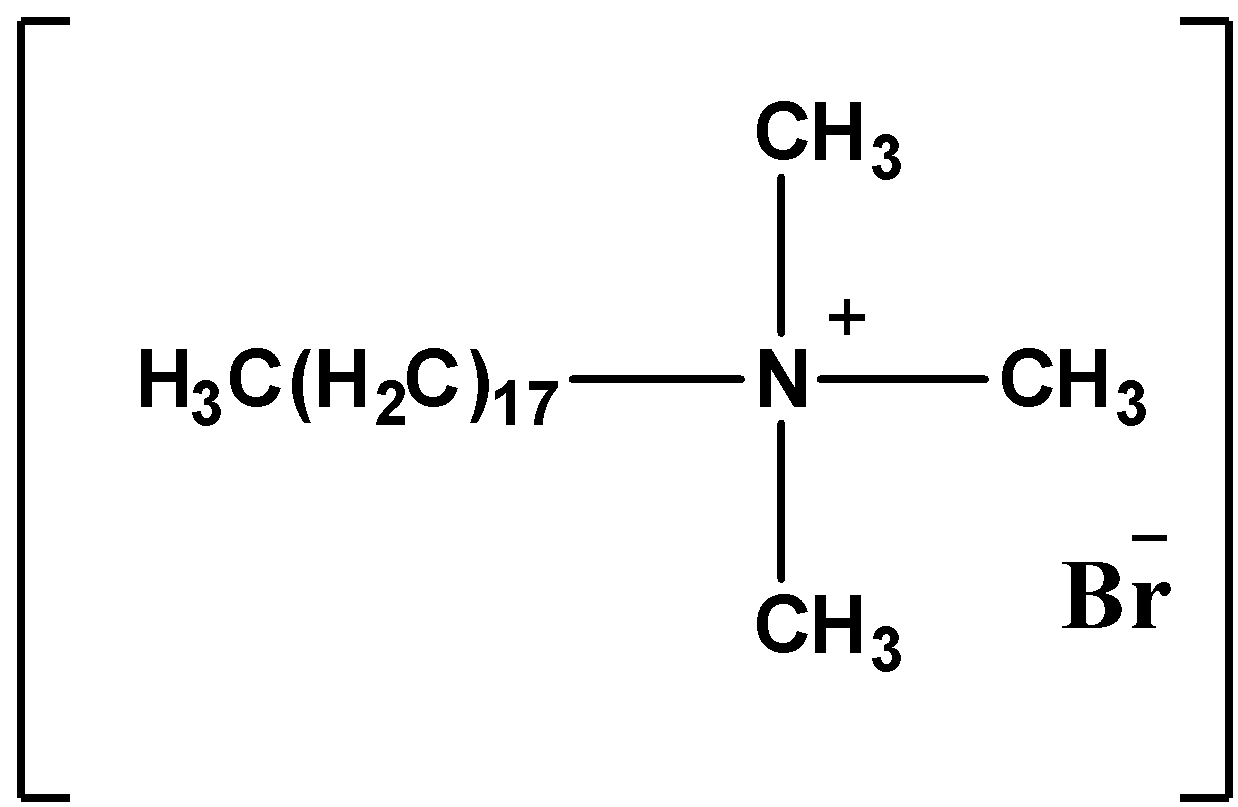
Trimethyl stearyl ammonium bromide contains quaternary ammonium salt with an active cation part on the surface of the molecule. So, Trimethyl stearyl ammonium bromide is invert soap.
Coming to option C, Ethoxylated nonylphenol, it is non-ionic in water and it is called non-ionic detergent. So, it is not correct.
Coming to option D, Sodium stearyl sulphate. The name itself says that it is anionic detergent because sodium is positively charged and stearyl sulphate is negatively charged means it has an anion active surface. So, it is not the correct option.
Therefore the correct answer for invert soap is Trimethyl stearyl ammonium bromide.
So, the correct option is B.
Note: Normal soap contains anion active surface but in case of invert soap, the cation part of the molecule is active. That is why normal soap is different from invert soap. Sodium stearyl sulphate is an example for normal soap.
Complete step by step solution:
Invert soap contains a surface which is positively charged, in normal soap a negatively charged surface is the active part.
Coming to the given options, option A, Pentaerythritol monostearate. The molecular formula of
Pentaerythritol monostearate is \[{{C}_{23}}{{H}_{46}}{{O}_{5}}\]. It does not have an active part on the surface of the molecule. So, it is not the correct option.
Coming to option B, Trimethyl stearyl ammonium bromide.
The structure of the Pentaerythritol monostearate is as follows.

Trimethyl stearyl ammonium bromide contains quaternary ammonium salt with an active cation part on the surface of the molecule. So, Trimethyl stearyl ammonium bromide is invert soap.
Coming to option C, Ethoxylated nonylphenol, it is non-ionic in water and it is called non-ionic detergent. So, it is not correct.
Coming to option D, Sodium stearyl sulphate. The name itself says that it is anionic detergent because sodium is positively charged and stearyl sulphate is negatively charged means it has an anion active surface. So, it is not the correct option.
Therefore the correct answer for invert soap is Trimethyl stearyl ammonium bromide.
So, the correct option is B.
Note: Normal soap contains anion active surface but in case of invert soap, the cation part of the molecule is active. That is why normal soap is different from invert soap. Sodium stearyl sulphate is an example for normal soap.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




