
Which of the following is electron deficient?
A.${(C{H_3})_2}$
B.${(Si{H_3})_2}$
C.${(B{H_3})_2}$
D.$P{H_3}$
Answer
581.1k+ views
Hint: The valence electrons present in carbon is four, the valence electrons present in silicon is four, the valence electron in boron is three and the valence electron in phosphorus is five.
Complete step by step answer:
The structure of ${(C{H_3})_2}$ is shown below.
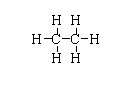
The atomic number of carbon is 6. The electronic configuration is $[He]2{s^2}2{p^2}$. Hence, only four electrons take part in bond formation.
In ${(C{H_3})_2}$, all the valency of carbon is fulfilled so there is no electron deficiency.
The structure of ${(Si{H_3})_2}$ is shown below.
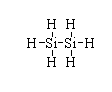
The atomic number of silicon is 14. The electronic configuration is $[Ne]3{s^2}3{p^2}$. Hence, the valence electron in silicon is four.
In ${(Si{H_3})_2}$, all the valency of silicon is fulfilled so there is no electron deficiency.
The structure of ${(B{H_3})_2}$ is shown below.
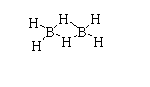
The atomic number of boron is 5. The electronic configuration is $[He]2{s^2}2{p^1}$. The valence electron in boron is 3.
In diborane, fourteen electrons are needed to form seven bonds but only twelve bonds are present. Due to this it forms a special type of structure.
Thus, diborane is electron deficient.
The structure of $P{H_3}$ is shown below.
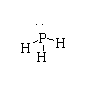
The atomic number of phosphorus is 17. The electronic configuration is $[Ne]3{s^2}3{p^3}$. There are five valence electrons in phosphorus.
In $P{H_3}$, three electrons take part in bonding and two nonbonding lone pairs are present.
Hence, in $P{H_3}$ all the valency is fulfilled, therefore it is not electron deficient.
Thus, the correct answer is option C.
Note:
When the compound boron hydride $B{H_3}$ undergoes dimerizes to form diborane${B_2}{H_6}$ , each boron atom contains only six electrons, thus its octet is incomplete.
Complete step by step answer:
The structure of ${(C{H_3})_2}$ is shown below.

The atomic number of carbon is 6. The electronic configuration is $[He]2{s^2}2{p^2}$. Hence, only four electrons take part in bond formation.
In ${(C{H_3})_2}$, all the valency of carbon is fulfilled so there is no electron deficiency.
The structure of ${(Si{H_3})_2}$ is shown below.
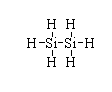
The atomic number of silicon is 14. The electronic configuration is $[Ne]3{s^2}3{p^2}$. Hence, the valence electron in silicon is four.
In ${(Si{H_3})_2}$, all the valency of silicon is fulfilled so there is no electron deficiency.
The structure of ${(B{H_3})_2}$ is shown below.
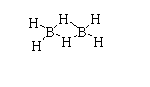
The atomic number of boron is 5. The electronic configuration is $[He]2{s^2}2{p^1}$. The valence electron in boron is 3.
In diborane, fourteen electrons are needed to form seven bonds but only twelve bonds are present. Due to this it forms a special type of structure.
Thus, diborane is electron deficient.
The structure of $P{H_3}$ is shown below.

The atomic number of phosphorus is 17. The electronic configuration is $[Ne]3{s^2}3{p^3}$. There are five valence electrons in phosphorus.
In $P{H_3}$, three electrons take part in bonding and two nonbonding lone pairs are present.
Hence, in $P{H_3}$ all the valency is fulfilled, therefore it is not electron deficient.
Thus, the correct answer is option C.
Note:
When the compound boron hydride $B{H_3}$ undergoes dimerizes to form diborane${B_2}{H_6}$ , each boron atom contains only six electrons, thus its octet is incomplete.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




