
Which of the following is an example of liquid dishwashing detergent?
A.${\rm{C}}{{\rm{H}}_{\rm{3}}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{2}}}} \right)_{{\rm{10}}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OS}}{{\rm{O}}_{\rm{3}}}^ - {\rm{N}}{{\rm{a}}^ + }$
B.
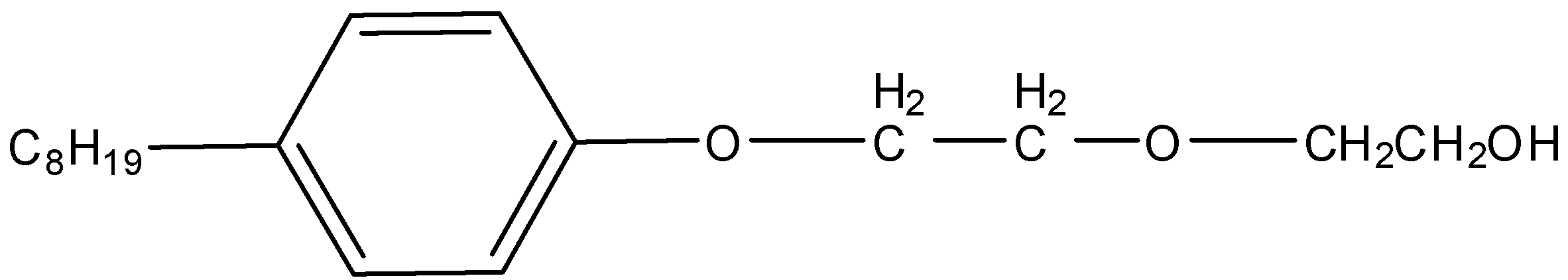
C.
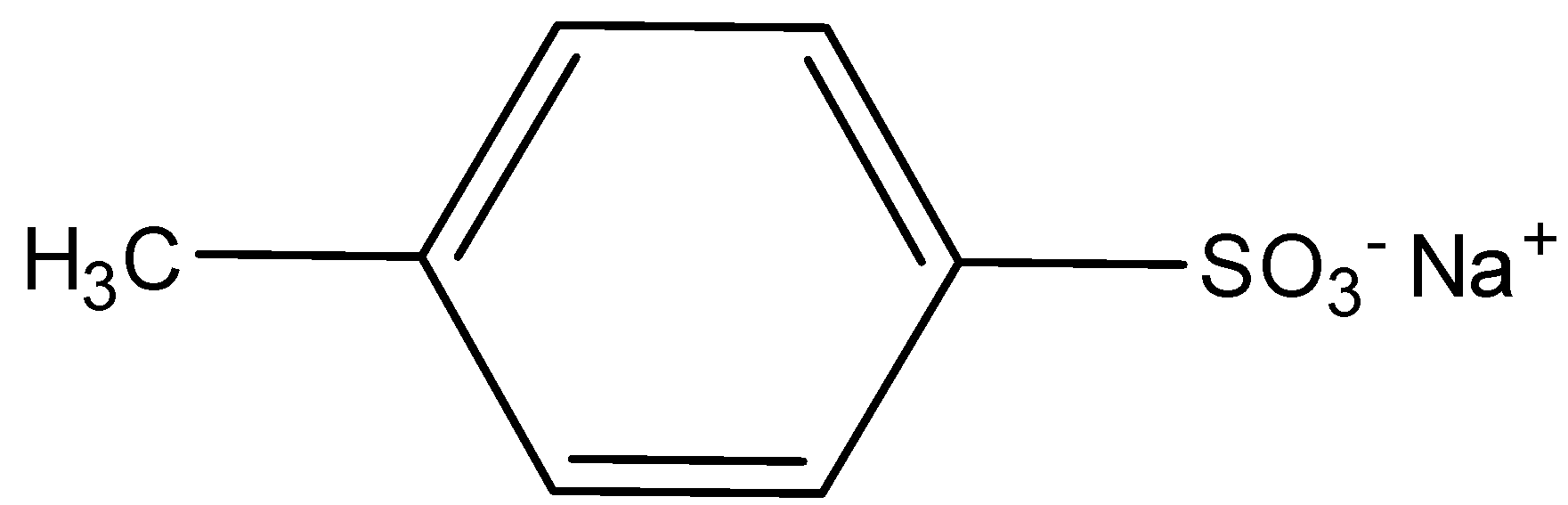
D.
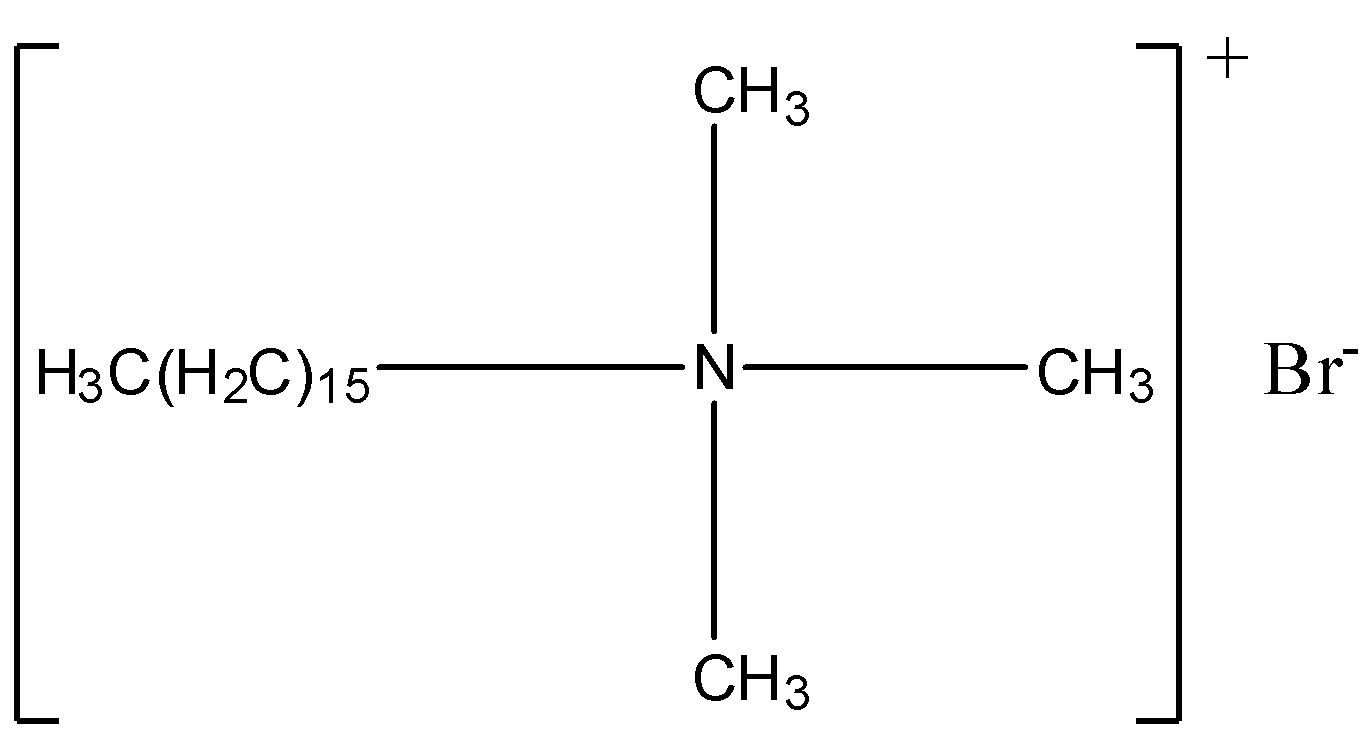



Answer
584.4k+ views
Hint: We know that synthetic detergents are also cleansing agents like soaps. They can be used in both hard and soft water because of their capacity to produce foam even in hard water. Some of the detergents produce foam even in ice cold water.
Complete step by step answer:
Let’s discuss the classification of synthetic detergents. They are of three types, cationic detergents, anionic detergents and non-ionic detergents.
Anionic detergents are those possessing sodium salts of sulfonated hydrocarbons (long chain) or alcohols. This type of detergent is prepared by neutralizing the reaction of alkyl hydrogen sulfates with alkali. Alkyl hydrogen sulfates can be prepared by reacting long chain alcohols with sulphuric acid (conc.) .This type of detergent is mainly used for household work. In toothpastes also this type of detergent is used. One example is,
${\rm{C}}{{\rm{H}}_{\rm{3}}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{2}}}} \right)_{{\rm{10}}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OS}}{{\rm{O}}_{\rm{3}}}^ - {\rm{N}}{{\rm{a}}^ + }$
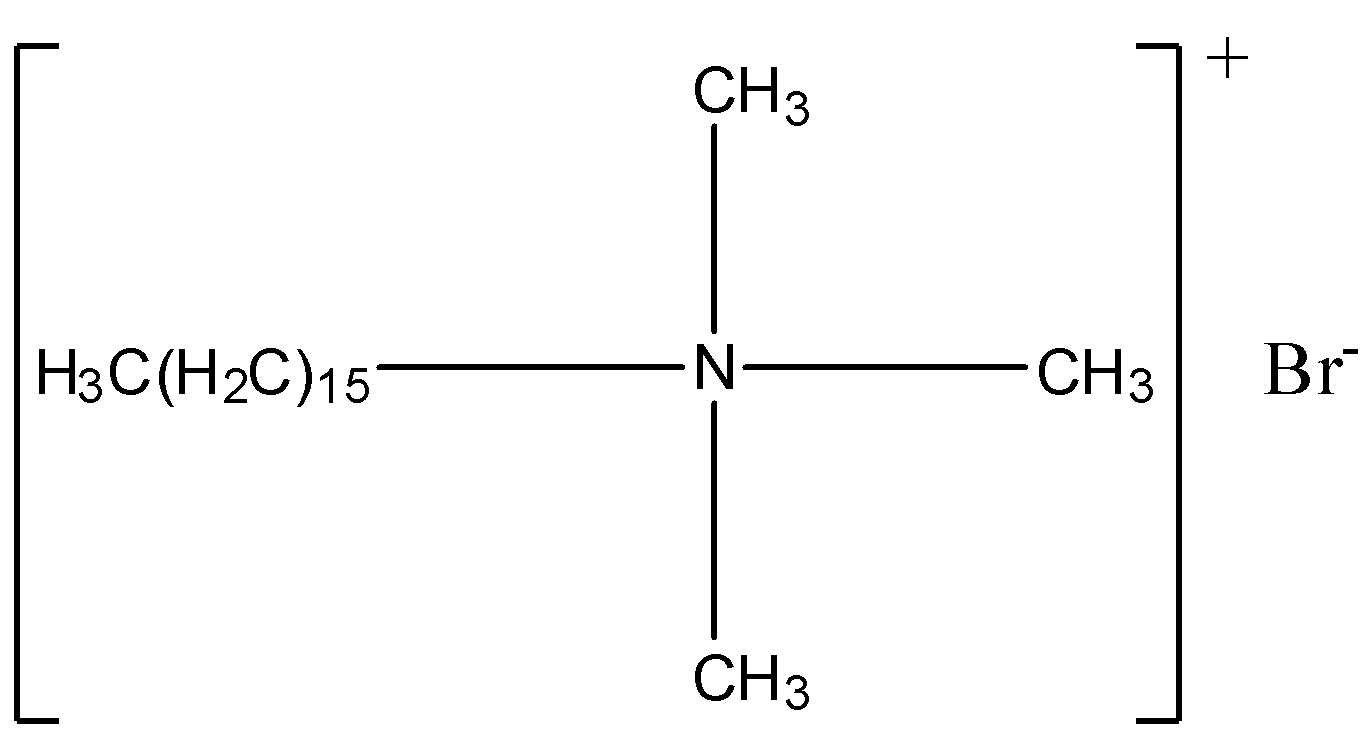
Cationic detergents are quaternary ammonium salts of amines with chlorides, acetates or bromides (anions). They are named as cationic detergents because their cationic part has a long chain of hydrocarbons and carries positive charge on N (nitrogen) atoms. One popular cationic detergent is cetyltrimethylammonium bromide which is used in conditioners. Its structure is,
Non-ionic detergents do not possess any ion in their composition, so they are known as non-ionic detergents. Liquid dishwashing detergents fall under this category of detergents. The cleansing action of this type of detergent is the same as that of soap.
Here, we have to identify the Liquid dishwashing detergents. We know that Liquid dishwashing detergents are non-ionic detergents. So, liquid dishwashing detergent is,
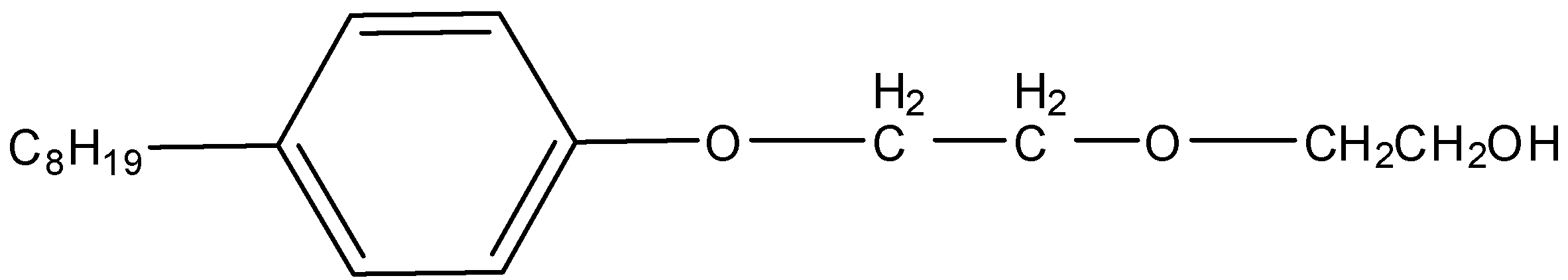
So, the correct answer is Option B.
Note:
The use of detergents has harmful effects on the environment as the bacteria cannot degrade the highly branched long hydrocarbon chain. Slow degradation results in the accumulation of detergents. Effluents having such detergent reach the water bodies and pollute the water.
Complete step by step answer:
Let’s discuss the classification of synthetic detergents. They are of three types, cationic detergents, anionic detergents and non-ionic detergents.
Anionic detergents are those possessing sodium salts of sulfonated hydrocarbons (long chain) or alcohols. This type of detergent is prepared by neutralizing the reaction of alkyl hydrogen sulfates with alkali. Alkyl hydrogen sulfates can be prepared by reacting long chain alcohols with sulphuric acid (conc.) .This type of detergent is mainly used for household work. In toothpastes also this type of detergent is used. One example is,
${\rm{C}}{{\rm{H}}_{\rm{3}}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{2}}}} \right)_{{\rm{10}}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OS}}{{\rm{O}}_{\rm{3}}}^ - {\rm{N}}{{\rm{a}}^ + }$
Cationic detergents are quaternary ammonium salts of amines with chlorides, acetates or bromides (anions). They are named as cationic detergents because their cationic part has a long chain of hydrocarbons and carries positive charge on N (nitrogen) atoms. One popular cationic detergent is cetyltrimethylammonium bromide which is used in conditioners. Its structure is,

Non-ionic detergents do not possess any ion in their composition, so they are known as non-ionic detergents. Liquid dishwashing detergents fall under this category of detergents. The cleansing action of this type of detergent is the same as that of soap.
Here, we have to identify the Liquid dishwashing detergents. We know that Liquid dishwashing detergents are non-ionic detergents. So, liquid dishwashing detergent is,

So, the correct answer is Option B.
Note:
The use of detergents has harmful effects on the environment as the bacteria cannot degrade the highly branched long hydrocarbon chain. Slow degradation results in the accumulation of detergents. Effluents having such detergent reach the water bodies and pollute the water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




