
Which of the following is an antiaromatic compound?
A.
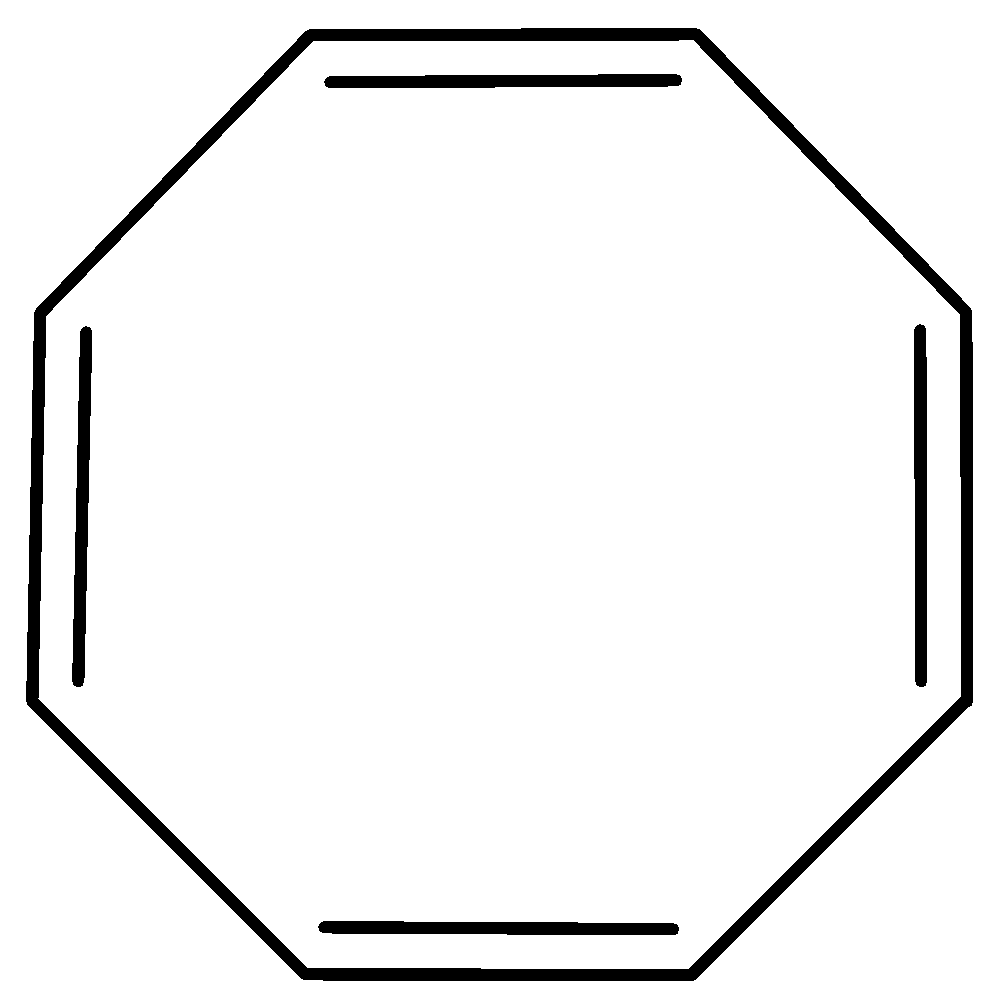
B.
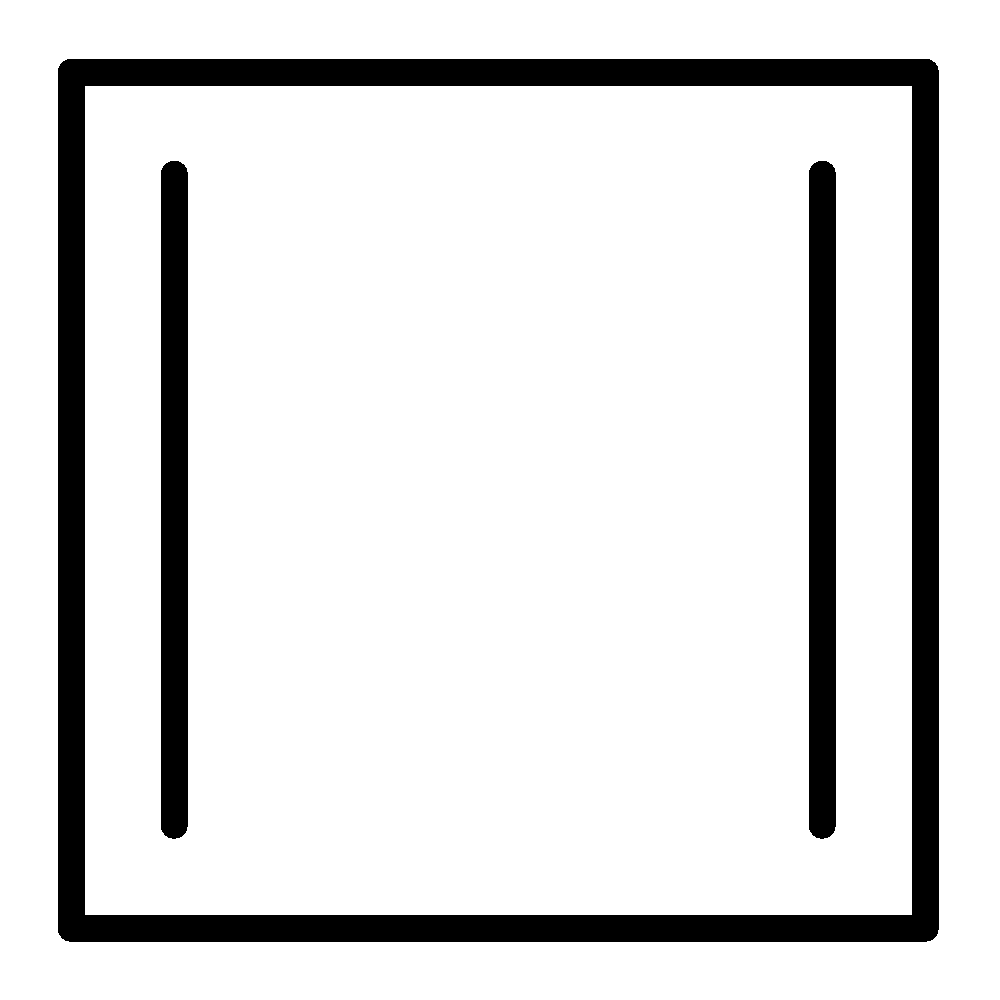
C.
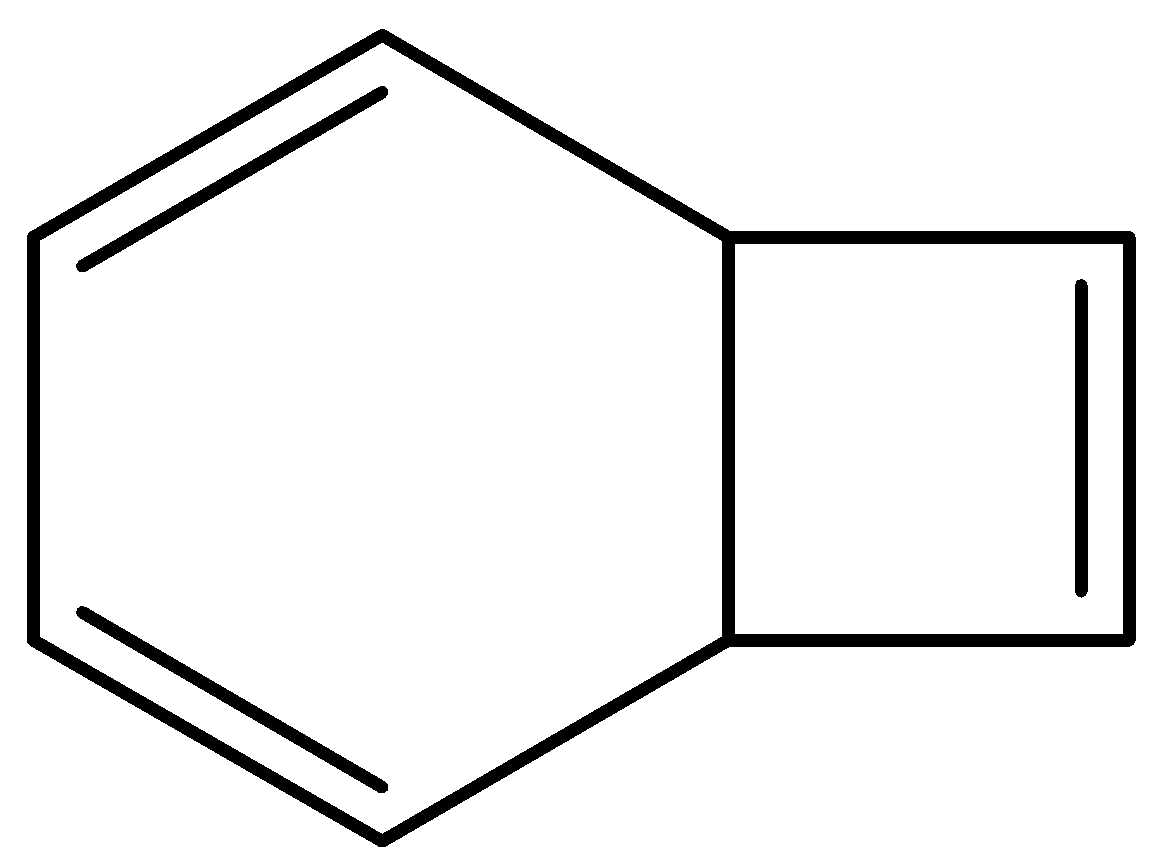
D.None of these



Answer
556.8k+ views
Hint: We must remember that the aromaticity is one amongst the foremost widely used concepts in chemistry. It was first alone applied to organic cyclic hydrocarbons. In 1931, Hückel came up together with his famous formula to characterize aromatic compounds, which is popularly called Hückel's $\left( {4n + 2} \right)\pi $ -electron rule.
Complete step by step answer:
We know that an aromatic compound must follow the below criteria:
1.Compounds must be cyclic and it has some number of conjugated $\pi $ bonds.
2.The atoms in the ring usually have an unhybridized $p$ orbital.
3.The unhybridized $p$ orbital necessarily overlaps to make an endless ring of parallel orbitals. The structure of that compound must be planar or nearly planar, due to an effective overlap to occur.
4.The delocalization of the $\pi $ electrons must decrease electronic energy over the ring.
For example: Benzene (Kekule’s structure$(cyclohexatriene)$)
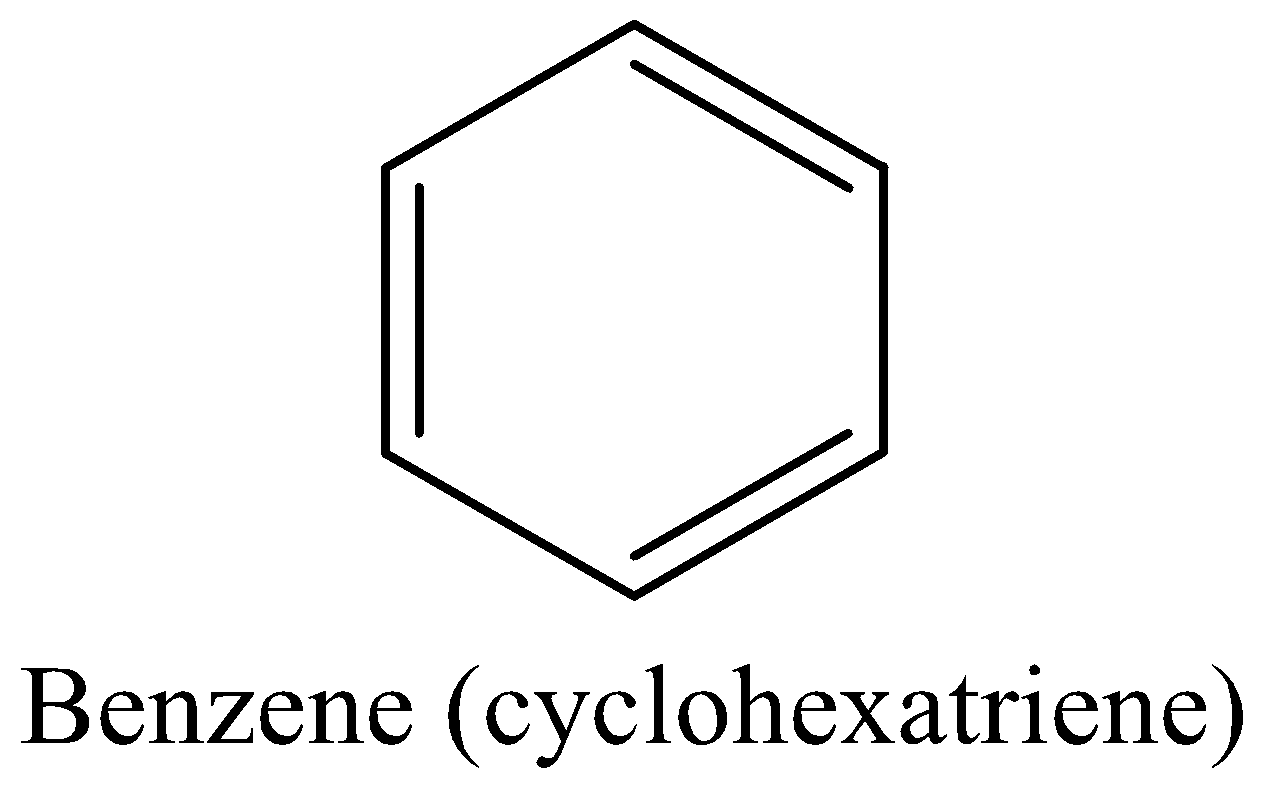
We must know that the benzene is aromatic and more stable, it meets all the above four criteria.
If the number of $\pi $ electrons in the system is $(4n)$, that system is anti-aromatic.

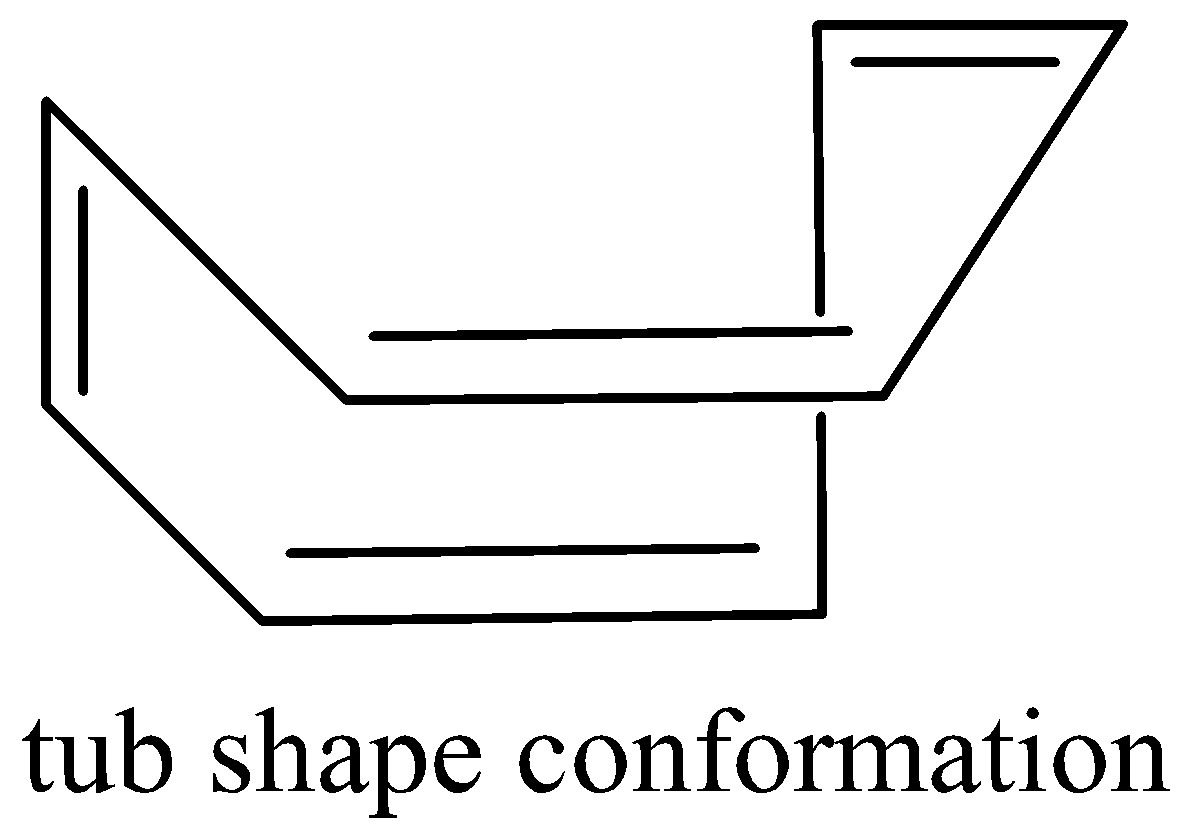
It escapes its aromaticity by abandoning planar geometry. In fact, it’s tub-shaped structure during which each covalent bond is nearly perpendicular to its neighboring double bond, thereby reducing the $\pi - $overlap to an excellent extent. Cyclooctatetraene is non-aromatic.
Cyclooctatetraene tub shape is as follows,
Cyclobutadiene
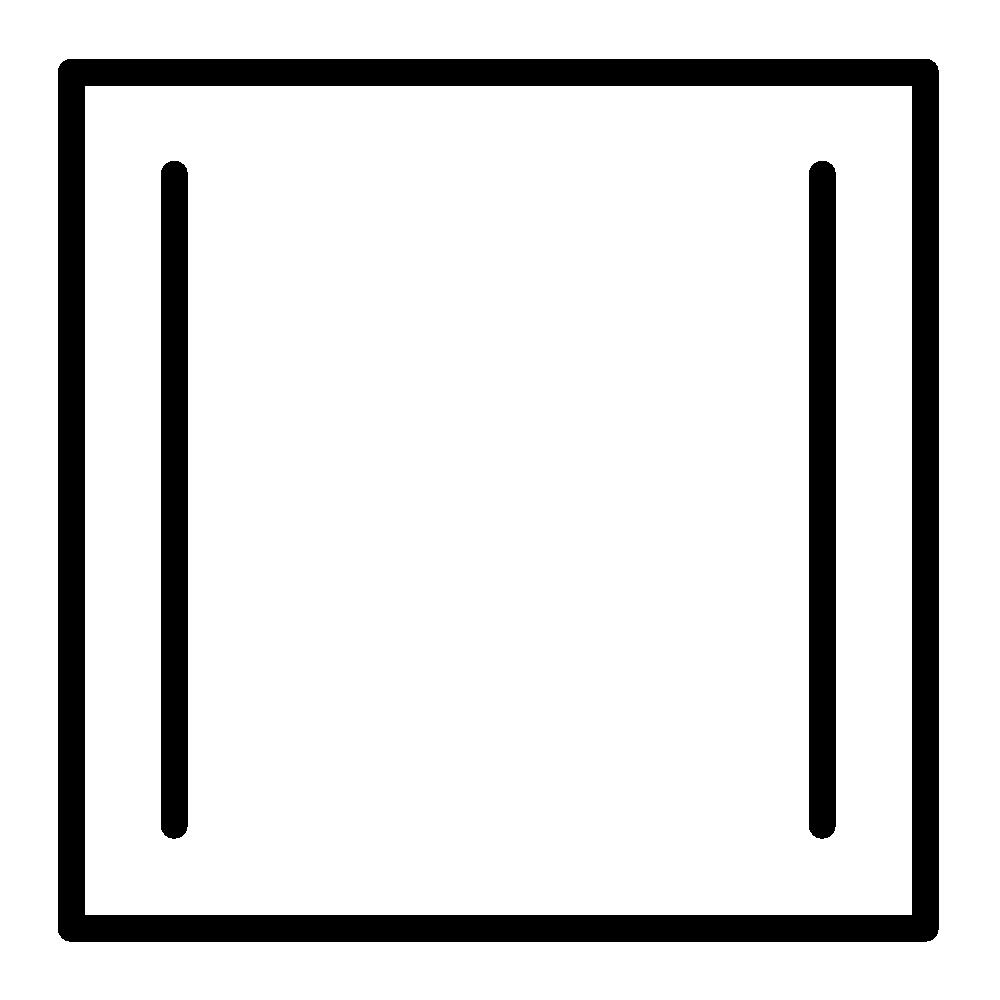
Cyclobutadiene follows the first three criteria listed above. Delocalization of the $\pi - $ electrons increases cyclobutadiene electronic energy. So, this is less stable. Cyclobutadiene is planar. It is $4\pi $ electrons system, it is an anti-aromatic.
\[bicyclo[4.2.0]octa - 2,4,7 - triene\] structure is as follows,
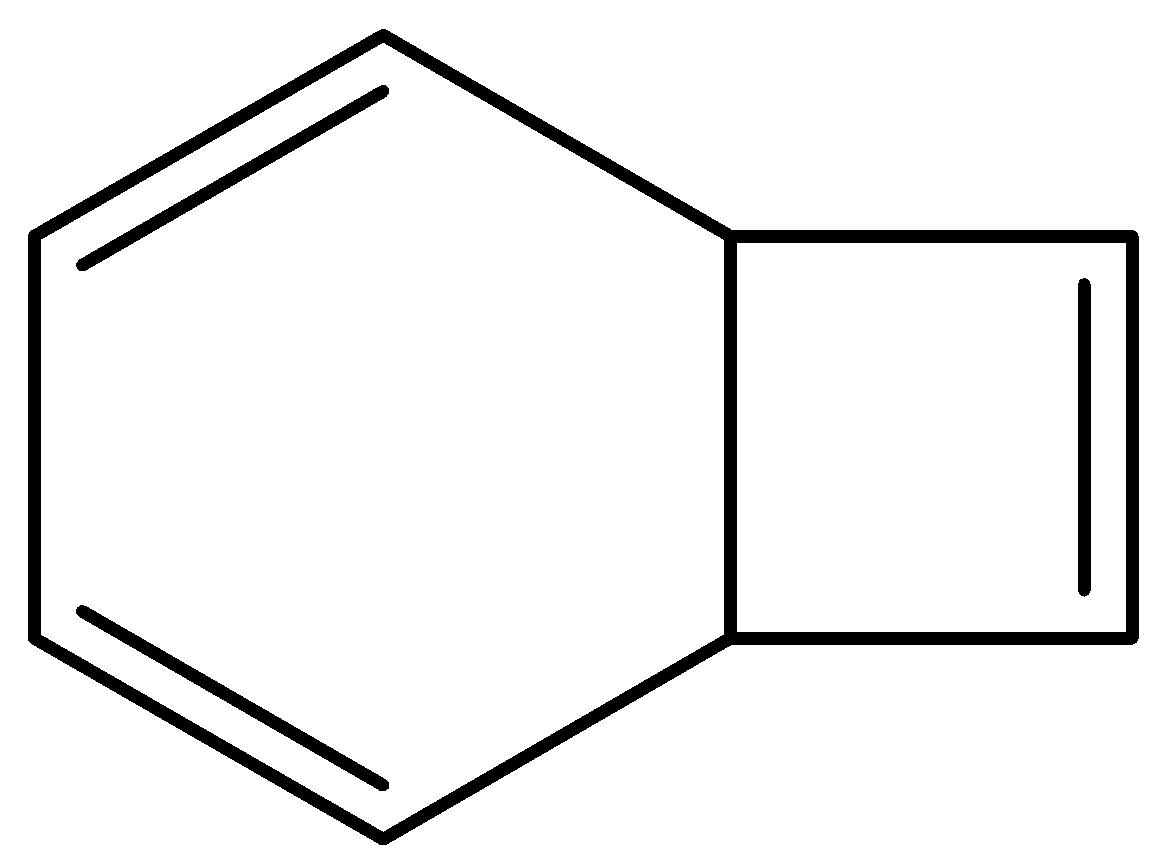
This is not planar. And it contains $s{p^3}$ hybridized carbon.

So, this is non-aromatic.
Therefore, option B is the correct answer and it is an anti-aromatic.
Note:
We need to remember that the aromaticity is vital because aromaticity makes molecules more stable. Aromatic compounds have high resonance, this high resonance makes their additional stable. And in biochemistry and in industry aromaticity plays an important role. Some compounds do not contain benzene rings but show aromatic characters, they are called as non benzenoid aromatic compounds.
Complete step by step answer:
We know that an aromatic compound must follow the below criteria:
1.Compounds must be cyclic and it has some number of conjugated $\pi $ bonds.
2.The atoms in the ring usually have an unhybridized $p$ orbital.
3.The unhybridized $p$ orbital necessarily overlaps to make an endless ring of parallel orbitals. The structure of that compound must be planar or nearly planar, due to an effective overlap to occur.
4.The delocalization of the $\pi $ electrons must decrease electronic energy over the ring.
For example: Benzene (Kekule’s structure$(cyclohexatriene)$)

We must know that the benzene is aromatic and more stable, it meets all the above four criteria.
If the number of $\pi $ electrons in the system is $(4n)$, that system is anti-aromatic.

It escapes its aromaticity by abandoning planar geometry. In fact, it’s tub-shaped structure during which each covalent bond is nearly perpendicular to its neighboring double bond, thereby reducing the $\pi - $overlap to an excellent extent. Cyclooctatetraene is non-aromatic.
Cyclooctatetraene tub shape is as follows,

Cyclobutadiene

Cyclobutadiene follows the first three criteria listed above. Delocalization of the $\pi - $ electrons increases cyclobutadiene electronic energy. So, this is less stable. Cyclobutadiene is planar. It is $4\pi $ electrons system, it is an anti-aromatic.
\[bicyclo[4.2.0]octa - 2,4,7 - triene\] structure is as follows,

This is not planar. And it contains $s{p^3}$ hybridized carbon.

So, this is non-aromatic.
Therefore, option B is the correct answer and it is an anti-aromatic.
Note:
We need to remember that the aromaticity is vital because aromaticity makes molecules more stable. Aromatic compounds have high resonance, this high resonance makes their additional stable. And in biochemistry and in industry aromaticity plays an important role. Some compounds do not contain benzene rings but show aromatic characters, they are called as non benzenoid aromatic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




