
Which of the following is a tricarboxylic acid?
(A)Citric acid
(B)Malonic acid
(C)Succinic acid
(D)Malic acid
Answer
533.4k+ views
Hint:As we know that carboxylic acid is an organic acid which contains a carboxyl group (C(=O)OH) attached to an R-group (R = alkyl or aryl). Tricarboxylic acid, by the name itself, says that it is a category of carboxylic acid which has 3 C(=O)OH group and hence we will draw the structure of each compound given in the options and check for 3 C(=O)OH group.
Complete step-by-step answer:-Carboxylic acid are one of the class of organic compounds in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and to a hydroxyl group (―OH) by a single bond i.e., its functional group represented as C(=O)OH.
-Carboxylic acids occur widely in nature and its derivatives are of utmost importance in various chemical reactions.
-Tricarboxylic acids belongs to the class of carboxylic acids which contains 3 carboxyl groups (C(=O)OH) attached to R-groups (R = alkyl or aryl).
Now let us draw structure of each acid compound given in the options:-
(A)Citric acid:
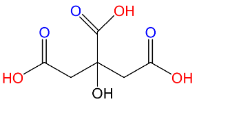
As we can see that this compound carries 3 carboxyl groups (C(=O)OH) attached to the parent chain. Hence this is a tricarboxylic acid. Citric acid is a weak acid which naturally occurs in citric fruits.
(B)Malonic acid:
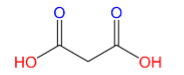
Here only 2 carboxyl groups (C(=O)OH) are present, therefore Malonic acid is a dicarboxylic acid.
(C)Succinic acid:
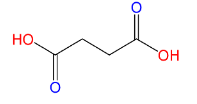
Here also only 2 carboxyl groups (C(=O)OH) are present, therefore Succinic acid is a dicarboxylic acid.
(D)Malic acid:
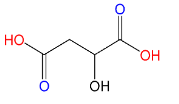
Here also only 2 carboxyl groups (C(=O)OH) are present, therefore Malic acid is a dicarboxylic acid.
Therefore the correct option is (A) Citric acid.
Note:-Carboxylic acids generally have higher melting point than other hydrocarbons and functional groups because of its intermolecular hydrogen bonding. And since Tricarboxylic acids have 3 carboxyl groups (C(=O)OH) attached to R-groups, it do have the ability to form strong hydrogen bonds and this results in their high boiling points.
-As the number of carboxyl group increases in the compound, nomenclature changes as follows:-
1 (C(=O)OH) group : monocarboxylic acid (or simply carboxylic acid)
2 (C(=O)OH) group : dicarboxylic acid
3 (C(=O)OH) group : tricarboxylic acid
4 (C(=O)OH) group : tetracarboxylic acid
and so on.
Complete step-by-step answer:-Carboxylic acid are one of the class of organic compounds in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond and to a hydroxyl group (―OH) by a single bond i.e., its functional group represented as C(=O)OH.
-Carboxylic acids occur widely in nature and its derivatives are of utmost importance in various chemical reactions.
-Tricarboxylic acids belongs to the class of carboxylic acids which contains 3 carboxyl groups (C(=O)OH) attached to R-groups (R = alkyl or aryl).
Now let us draw structure of each acid compound given in the options:-
(A)Citric acid:

As we can see that this compound carries 3 carboxyl groups (C(=O)OH) attached to the parent chain. Hence this is a tricarboxylic acid. Citric acid is a weak acid which naturally occurs in citric fruits.
(B)Malonic acid:

Here only 2 carboxyl groups (C(=O)OH) are present, therefore Malonic acid is a dicarboxylic acid.
(C)Succinic acid:

Here also only 2 carboxyl groups (C(=O)OH) are present, therefore Succinic acid is a dicarboxylic acid.
(D)Malic acid:

Here also only 2 carboxyl groups (C(=O)OH) are present, therefore Malic acid is a dicarboxylic acid.
Therefore the correct option is (A) Citric acid.
Note:-Carboxylic acids generally have higher melting point than other hydrocarbons and functional groups because of its intermolecular hydrogen bonding. And since Tricarboxylic acids have 3 carboxyl groups (C(=O)OH) attached to R-groups, it do have the ability to form strong hydrogen bonds and this results in their high boiling points.
-As the number of carboxyl group increases in the compound, nomenclature changes as follows:-
1 (C(=O)OH) group : monocarboxylic acid (or simply carboxylic acid)
2 (C(=O)OH) group : dicarboxylic acid
3 (C(=O)OH) group : tricarboxylic acid
4 (C(=O)OH) group : tetracarboxylic acid
and so on.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




