
Which of the following have only ${2^0}$ H atom :
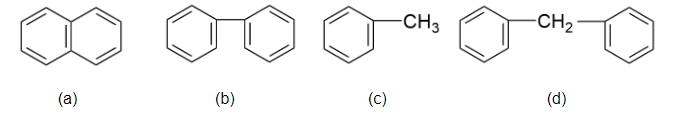
(A)- a and b
(B)- a, b and d
(C)- a, c and d
(D)- a, b, c and d

Answer
573.3k+ views
Hint: To find the degree of hydrogen in the given four organic compounds. First you have to find the number of primary, secondary and tertiary carbons and then you will get the desired secondary hydrogens.
Complete step by step answer:
Before finding out the number of ${2^0}$ hydrogen, we have to understand the meaning of the degree of carbon.
In Organic chemists with carbon chemistry as their subject, it has developed all kinds of shorthand phrases to describe structures and phenomena that might otherwise take a sentence of two to explain. In this question it is the terminology of primary, secondary, tertiary carbon. quaternary.
- Primary carbons: They are carbons attached to only one other carbon. (Hydrogens – although usually 3 in number in this case – are ignored in this terminology). This primary carbon can be written as ${1^0}$ (1 with a degree symbol) has one carbon attached to this carbon atom. In this case, the maximum number of hydrogens attached to it is 3.
- Secondary carbons: They are carbons attached to two other carbons. A secondary carbon written as ${2^0}$ (2 with a degree symbol) is a carbon attached to two other carbons. In this case, the maximum number of hydrogens attached to it is 2.
- Tertiary carbons: They are carbons which are attached to three other carbons. A tertiary carbon written as ${3^0}$ (3 with a degree symbol) is a carbon attached to three other carbons. In this case, the maximum number of hydrogens attached to it is 1.
- Quaternary carbons: Finally, quaternary carbons are attached to four other carbons. A quaternary carbon written as ${4^0}$ (4 with a degree symbol) is a carbon attached to four other carbons. In this case, there is no hydrogen attached to the carbon.
Now, the terminology for one, two- and three-degree hydrogens are listed below:
- ${1^0}$ Hydrogen: All those hydrogens which are attached to primary carbon i.e. ${1^0}$ carbon atoms are termed as ${1^0}$ hydrogens.
- ${2^0}$ Hydrogen: All those hydrogens which are attached to secondary carbon i.e. ${2^0}$ carbon atoms are termed as ${2^0}$ hydrogens.
-$3^0$ Hydrogen: All those hydrogens which are attached to tertiary carbon i.e. $3^0$ carbon atoms are termed as $3^0$ hydrogens.
With reference to the above discussion, we can say that only figure a, b and d have hydrogen atoms.
Therefore, the correct answer of the above question is option (B)- a, b and d.
Note: Don't think that this nomenclature (primary, secondary and tertiary) is only for carbon and hydrogen. Just like carbons and hydrogens, we can also describe other functional groups as primary secondary and tertiary. For example, an alcohol group attached to a primary carbon is also termed as primary alcohol.
Complete step by step answer:
Before finding out the number of ${2^0}$ hydrogen, we have to understand the meaning of the degree of carbon.
In Organic chemists with carbon chemistry as their subject, it has developed all kinds of shorthand phrases to describe structures and phenomena that might otherwise take a sentence of two to explain. In this question it is the terminology of primary, secondary, tertiary carbon. quaternary.
- Primary carbons: They are carbons attached to only one other carbon. (Hydrogens – although usually 3 in number in this case – are ignored in this terminology). This primary carbon can be written as ${1^0}$ (1 with a degree symbol) has one carbon attached to this carbon atom. In this case, the maximum number of hydrogens attached to it is 3.
- Secondary carbons: They are carbons attached to two other carbons. A secondary carbon written as ${2^0}$ (2 with a degree symbol) is a carbon attached to two other carbons. In this case, the maximum number of hydrogens attached to it is 2.
- Tertiary carbons: They are carbons which are attached to three other carbons. A tertiary carbon written as ${3^0}$ (3 with a degree symbol) is a carbon attached to three other carbons. In this case, the maximum number of hydrogens attached to it is 1.
- Quaternary carbons: Finally, quaternary carbons are attached to four other carbons. A quaternary carbon written as ${4^0}$ (4 with a degree symbol) is a carbon attached to four other carbons. In this case, there is no hydrogen attached to the carbon.
Now, the terminology for one, two- and three-degree hydrogens are listed below:
- ${1^0}$ Hydrogen: All those hydrogens which are attached to primary carbon i.e. ${1^0}$ carbon atoms are termed as ${1^0}$ hydrogens.
- ${2^0}$ Hydrogen: All those hydrogens which are attached to secondary carbon i.e. ${2^0}$ carbon atoms are termed as ${2^0}$ hydrogens.
-$3^0$ Hydrogen: All those hydrogens which are attached to tertiary carbon i.e. $3^0$ carbon atoms are termed as $3^0$ hydrogens.
With reference to the above discussion, we can say that only figure a, b and d have hydrogen atoms.
Therefore, the correct answer of the above question is option (B)- a, b and d.
Note: Don't think that this nomenclature (primary, secondary and tertiary) is only for carbon and hydrogen. Just like carbons and hydrogens, we can also describe other functional groups as primary secondary and tertiary. For example, an alcohol group attached to a primary carbon is also termed as primary alcohol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




