
Which of the following have a glycosidic bond and hydrogen bond respectively?
A. Polysaccharide and water
B. Fat and Polysaccharide
C. Protein and Fat
D. Fat and Protein
Answer
583.2k+ views
Hint: A glycosidic bond is a bond between the sugar molecule and to another molecule while the hydrogen bond is a type of bond that exists between the hydrogen atom and a highly electronegative element.
Complete answer:
Polysaccharides are a long chain molecule made up of more than ten monomeric units of monosaccharides. These monosaccharide units are held by the glycosidic bond. It is the most abundant molecule present in food.
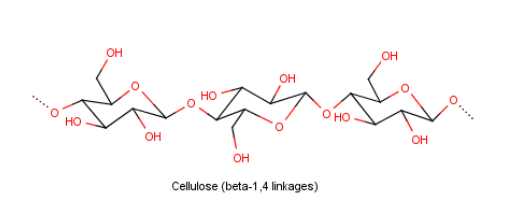
For example- Cellulose – it is made up of glucose units. These glucose units are held together by $\beta (1 \to 4)$ glycosidic bond.
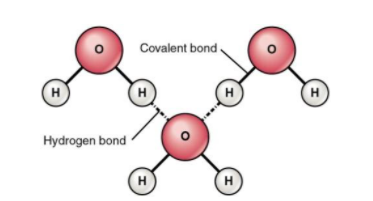
Water molecules have one oxygen atom and two hydrogen atoms. Two water molecules are held together by the hydrogen bonds. This is known as oxygen-hydrogen bonding. The hydrogen atom that has a plus end is associated with the oxygen atom that has a minus end. The liquid state of water and high solubility in water is due to the presence of hydrogen bonds.
Protein is made up of several amino acids. These amino acids are joined together by a peptide bond. In the case of fat, the ester bonds are present.
Therefore the correct answer is option A that is Polysaccharide and water.
Note: A glycosidic bond is a type of covalent chemical bonds that hold together a glycoside. The glycoside is simple sugar molecules that are attached to other molecules. Glycosidic bonds can be O-linked or N-linked bonds.
Hydrogen bonding is formed between the hydrogen atom and electronegative atom like oxygen, chlorine, etc Hydrogen bonds can be intermolecular and intramolecular. When hydrogen bond is existing between the two different molecules is known as an intermolecular bond, whereas when hydrogen bond is formed within the two atoms of molecule are called intramolecular.
Complete answer:
Polysaccharides are a long chain molecule made up of more than ten monomeric units of monosaccharides. These monosaccharide units are held by the glycosidic bond. It is the most abundant molecule present in food.
For example- Cellulose – it is made up of glucose units. These glucose units are held together by $\beta (1 \to 4)$ glycosidic bond.

Water molecules have one oxygen atom and two hydrogen atoms. Two water molecules are held together by the hydrogen bonds. This is known as oxygen-hydrogen bonding. The hydrogen atom that has a plus end is associated with the oxygen atom that has a minus end. The liquid state of water and high solubility in water is due to the presence of hydrogen bonds.

Protein is made up of several amino acids. These amino acids are joined together by a peptide bond. In the case of fat, the ester bonds are present.
Therefore the correct answer is option A that is Polysaccharide and water.
Note: A glycosidic bond is a type of covalent chemical bonds that hold together a glycoside. The glycoside is simple sugar molecules that are attached to other molecules. Glycosidic bonds can be O-linked or N-linked bonds.
Hydrogen bonding is formed between the hydrogen atom and electronegative atom like oxygen, chlorine, etc Hydrogen bonds can be intermolecular and intramolecular. When hydrogen bond is existing between the two different molecules is known as an intermolecular bond, whereas when hydrogen bond is formed within the two atoms of molecule are called intramolecular.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




