
Which of the following forms gives osazones different from the other three?
(A) Fructose
(B) Glucose
(C) Galactose
(D) Mannose
Answer
596.1k+ views
Hint: Osazone is the derivative of carbohydrate which is obtained by its reaction with phenylhydrazine in 1:3 ratio. Fructose is a ketohexose and has a keto group at C-2. Galactose and Mannose are epimers of Glucose.
Complete step by step solution:
Osazones are the derivatives of carbohydrates which are produced by their reaction with Phenylhydrazine. Total 3 moles of phenylhydrazine react with one mole of sugar to give one mole of phenylhydrazine. Let’s see the reactions of all of them to find which of the given sugar gives different osazones.
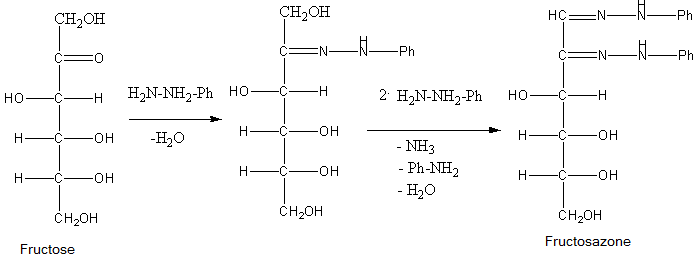
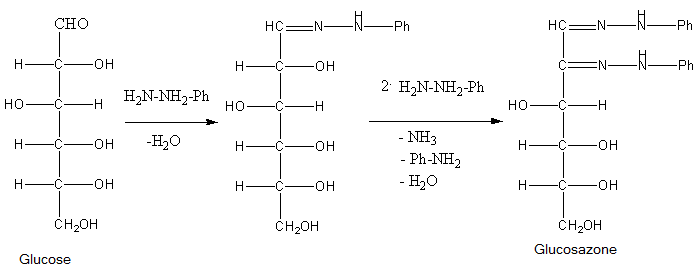
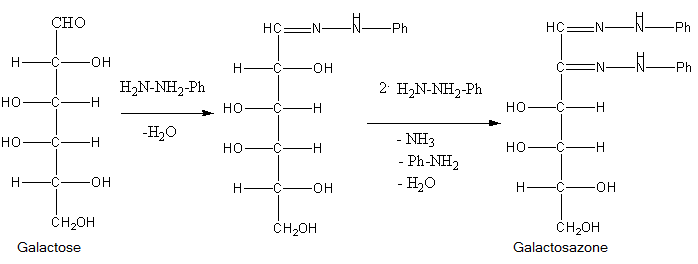
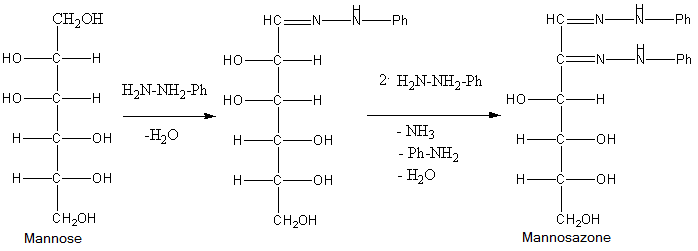
- Phenylhydrazine reacts with a carbonyl group of sugar to form phenylhydrazone and water. Then one molecule of phenylhydrazine reacts with phenylhydrazine and oxidises the \[\alpha \]-carbon and then this oxidised carbonyl group further reacts with phenylhydrazine to give osazone.
So, from the structures of the osazones formed by glucose, galactose, fructose and mannose, we can say that osazones of glucose, mannose and fructose are same and osazone formed by Galactose is different at stereochemistry at \[{4^{th}}\] carbon.
- This occurs because \[{1^{st}}\] and \[{2^{nd}}\] carbon of all the sugars react with phenylhydrazine to give osazones but only galactose of them has different stereochemistry at \[{4^{th}}\] carbon atom.
So, the correct answer is (C) Galactose.
Note: Do not just blindly think that as only fructose is ketohexose from the given sugars, it will form different osazone, actually the mechanism of reaction is such that only the sugars that have different structure at C-4, C-5 and C-6 will give different osazone amongst hexoses.
Complete step by step solution:
Osazones are the derivatives of carbohydrates which are produced by their reaction with Phenylhydrazine. Total 3 moles of phenylhydrazine react with one mole of sugar to give one mole of phenylhydrazine. Let’s see the reactions of all of them to find which of the given sugar gives different osazones.




- Phenylhydrazine reacts with a carbonyl group of sugar to form phenylhydrazone and water. Then one molecule of phenylhydrazine reacts with phenylhydrazine and oxidises the \[\alpha \]-carbon and then this oxidised carbonyl group further reacts with phenylhydrazine to give osazone.
So, from the structures of the osazones formed by glucose, galactose, fructose and mannose, we can say that osazones of glucose, mannose and fructose are same and osazone formed by Galactose is different at stereochemistry at \[{4^{th}}\] carbon.
- This occurs because \[{1^{st}}\] and \[{2^{nd}}\] carbon of all the sugars react with phenylhydrazine to give osazones but only galactose of them has different stereochemistry at \[{4^{th}}\] carbon atom.
So, the correct answer is (C) Galactose.
Note: Do not just blindly think that as only fructose is ketohexose from the given sugars, it will form different osazone, actually the mechanism of reaction is such that only the sugars that have different structure at C-4, C-5 and C-6 will give different osazone amongst hexoses.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




