
Which of the following form cationic micelles above certain concentrations?
(A) Sodium dodecyl sulphate
(B) Urea
(C) Sodium acetate
(D) Cetyl trimethyl ammonium bromide.
Answer
584.7k+ views
Hint: Micelles are formed by the aggregation of dispersed particles above a certain concentration. Polymeric micelles have a lower critical micelle concentration. Quaternary ammonium compounds form cationic surfactants. Use this to solve the given question.
Complete step by step answer:
Before answering this question, we have to understand what micelles are.
Micelles are basically loosely bound assembly of several ions of the surfactant which leads to the formation of the colloidal suspension. Micelles are formed by assembly of amphiphilic molecules. Amphiphilic nature is observed between two organic solvents or water and an organic solvent.
The hydrophilic head is surrounded by the solvent and the hydrophilic tail is in the middle of the micelle centre.
The hydration of the lipid head leads to the formation of micelle and this is known as normal phase micelle. Whereas inverse phase micelles have head groups in the centre and the tails extend out. Micelle formation and stability is dependent upon the concentration.
Now, let us discuss the question given to us.
Firstly we have sodium dodecyl sulphate. When dissolved in water, it forms a hydrocarbon core and hydrophilic ionic surface. It is an anionic surfactant and thus this is not the correct answer.
Then we have urea. Urea does not form micelle but affects the concentration of micelle. Therefore, this option is incorrect.
Then we have sodium acetate. Sodium acetate acts as an electrolyte and not micelle therefore, this is not the correct answer either.
And lastly we have cetyltrimethylammonium bromide. It is also known as cetrimonium bromide and it is a quaternary ammonium surfactant. It forms a cationic micelle by formation of ammonium cation.
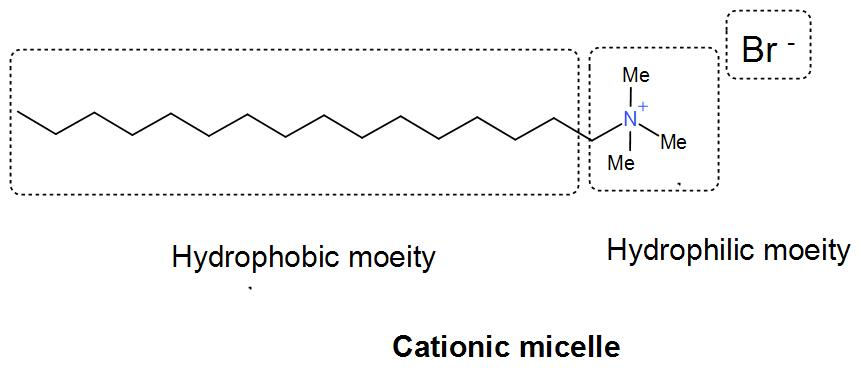
We can understand from the above discussion that cetyltrimethyl ammonium bromide forms cationic micelles.
Therefore, the correct answer is option (D) Cetyl trimethyl ammonium bromide.
Note: Micelle formation is important for absorption of fat soluble vitamin and lipids within the human body. It is also used for targeted drug delivery as rug nanoparticles. When surfactants are present above the Critical Micelle Concentration, they act as emulsifiers thus allowing a compound to dissolve in the solute in which it was insoluble before.
Complete step by step answer:
Before answering this question, we have to understand what micelles are.
Micelles are basically loosely bound assembly of several ions of the surfactant which leads to the formation of the colloidal suspension. Micelles are formed by assembly of amphiphilic molecules. Amphiphilic nature is observed between two organic solvents or water and an organic solvent.
The hydrophilic head is surrounded by the solvent and the hydrophilic tail is in the middle of the micelle centre.
The hydration of the lipid head leads to the formation of micelle and this is known as normal phase micelle. Whereas inverse phase micelles have head groups in the centre and the tails extend out. Micelle formation and stability is dependent upon the concentration.
Now, let us discuss the question given to us.
Firstly we have sodium dodecyl sulphate. When dissolved in water, it forms a hydrocarbon core and hydrophilic ionic surface. It is an anionic surfactant and thus this is not the correct answer.
Then we have urea. Urea does not form micelle but affects the concentration of micelle. Therefore, this option is incorrect.
Then we have sodium acetate. Sodium acetate acts as an electrolyte and not micelle therefore, this is not the correct answer either.
And lastly we have cetyltrimethylammonium bromide. It is also known as cetrimonium bromide and it is a quaternary ammonium surfactant. It forms a cationic micelle by formation of ammonium cation.

We can understand from the above discussion that cetyltrimethyl ammonium bromide forms cationic micelles.
Therefore, the correct answer is option (D) Cetyl trimethyl ammonium bromide.
Note: Micelle formation is important for absorption of fat soluble vitamin and lipids within the human body. It is also used for targeted drug delivery as rug nanoparticles. When surfactants are present above the Critical Micelle Concentration, they act as emulsifiers thus allowing a compound to dissolve in the solute in which it was insoluble before.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




