
Which of the following does not undergo alcohol condensation?
(A) $ HCHO $
(B) $ C{H_3}CHO $
(C) $ C{H_3}COC{H_3} $
(D) $ C{H_3}C{H_2}CHO $
Answer
498k+ views
Hint: Aldol condensation reaction is the characteristic reaction of all aldehyde and ketones which have at least one $ \alpha - $ hydrogen atom. Aldol condensation is carried out in the presence of dilute alkali in order to form $ \beta - $ hydroxy aldehyde or $ \beta - $ hydroxy ketone as a final product.
Complete Step By Step Answer:
During the aldol condensation reaction the $ \alpha - $ hydrogen atom of the compound reacts with hydroxide ion generated by alkali to form an enolate ion as an intermediate.
$ \alpha - $ hydrogen atom is an atom which is attached to an adjacent carbon of a functional group containing carbon.
The formed enolate ion further reacts with the carbonyl group of another molecule of aldehyde or ketone to form an anion. This anion further abstracts the proton from the water present as solvent to form the aldol or ketol.
As in the case of formaldehyde $ HCHO $ there is no carbon adjacent to the carbonyl carbon.
Therefore no $ \alpha - $ hydrogen atom is present in it. Hence, $ HCHO $ does not give an aldol condensation reaction.
As in the case of acetaldehyde $ C{H_3}CHO $ there is one carbon adjacent to carbonyl carbon and having three $ \alpha - $ hydrogen atoms. Therefore, it reacts in the presence of alkali to produce aldol.
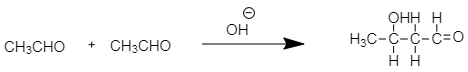
The final product formed by aldol condensation of acetaldehyde is $ 3 - $ hydroxy butanal.
As in the case of acetone there are two carbons adjacent to the carbonyl carbon and having total six $ \alpha - $ hydrogen atoms. Therefore, it reacts with alkali to produce the aldol.
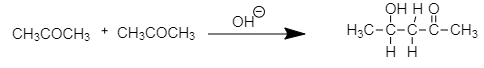
The final product formed by aldol condensation of acetone is $ 4 - $ hydroxy pentan- $ 2 - $ one.
Similarly in the case of propionaldehyde $ C{H_3}C{H_2}CHO $ there are two carbons adjacent to the carbonyl carbon and having total six $ \alpha - $ hydrogen atoms. Therefore, it reacts with alkali to produce the aldol.
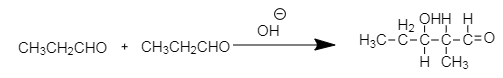
The final product formed by aldol condensation of propionaldehyde is $ 2 - $ methyl $ 3 - $ hydroxy pentanaldehyde.
$ \Rightarrow $ Formaldehyde does not give aldol condensation reaction.
Hence, option $ \left( i \right) $ is the correct option.
Note:
$ \beta - $ hydroxy aldehyde is commonly known as aldol and $ \beta - $ hydroxy ketone is commonly known as ketol. Aldol condensation is coming under the category of self-condensation reaction.
Complete Step By Step Answer:
During the aldol condensation reaction the $ \alpha - $ hydrogen atom of the compound reacts with hydroxide ion generated by alkali to form an enolate ion as an intermediate.
$ \alpha - $ hydrogen atom is an atom which is attached to an adjacent carbon of a functional group containing carbon.
The formed enolate ion further reacts with the carbonyl group of another molecule of aldehyde or ketone to form an anion. This anion further abstracts the proton from the water present as solvent to form the aldol or ketol.
As in the case of formaldehyde $ HCHO $ there is no carbon adjacent to the carbonyl carbon.
Therefore no $ \alpha - $ hydrogen atom is present in it. Hence, $ HCHO $ does not give an aldol condensation reaction.
As in the case of acetaldehyde $ C{H_3}CHO $ there is one carbon adjacent to carbonyl carbon and having three $ \alpha - $ hydrogen atoms. Therefore, it reacts in the presence of alkali to produce aldol.

The final product formed by aldol condensation of acetaldehyde is $ 3 - $ hydroxy butanal.
As in the case of acetone there are two carbons adjacent to the carbonyl carbon and having total six $ \alpha - $ hydrogen atoms. Therefore, it reacts with alkali to produce the aldol.

The final product formed by aldol condensation of acetone is $ 4 - $ hydroxy pentan- $ 2 - $ one.
Similarly in the case of propionaldehyde $ C{H_3}C{H_2}CHO $ there are two carbons adjacent to the carbonyl carbon and having total six $ \alpha - $ hydrogen atoms. Therefore, it reacts with alkali to produce the aldol.

The final product formed by aldol condensation of propionaldehyde is $ 2 - $ methyl $ 3 - $ hydroxy pentanaldehyde.
$ \Rightarrow $ Formaldehyde does not give aldol condensation reaction.
Hence, option $ \left( i \right) $ is the correct option.
Note:
$ \beta - $ hydroxy aldehyde is commonly known as aldol and $ \beta - $ hydroxy ketone is commonly known as ketol. Aldol condensation is coming under the category of self-condensation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




