
Which of the following does not form phenol or phenoxide?
A. Chlorobenzene
B. Benzoic acid
C. Benzene diazonium chloride
D. Sodium benzenesulfonate
Answer
373.8k+ views
Hint: Oxidation of an organic compound is the process of reaction of a compound without oxygen that happens in the presence of an oxidising agent. Phenol is an aromatic organic compound having the molecular formula C6H5OH.
Complete Step by Step Answer:
Phenol has the chemical formula \[{C_6}{H_5}OH\].
Phenoxides are salts of phenols.
They may be formed by reaction of phenols with a strong base.
It is also known as phenolates.
In this compound, a benzene ring is attached to an oxygen atom which bears a negative charge due to the loss of the proton.
We have to find out which of the following compounds doesn't form phenol or phenoxide.
A. Chlorobenzene
In this compound, a chlorine atom is attached to the benzene ring.
This compound on reaction with aqueous sodium hydroxide at 623K and at 300 atm pressure forms sodium phenoxide.
Sodium phenoxide reaction with dilute hydrochloric acid forms phenol.
The reaction happens as follows:
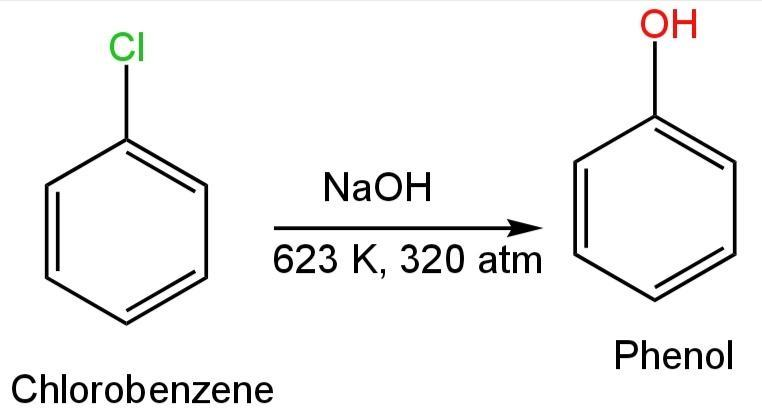
Image: Reaction of chlorobenzene with NaOH and HCl.
So, Chlorobenzene forms phenol and phenoxide ions.
So, A is incorrect.
B. Benzoic acid
Benzoic acid in the presence of any reagent or under any drastic conditions will not form phenol or phenoxide ions.
If this compound undergoes reduction, it will form benzyl alcohol not phenol.
The reaction will happen as follows:
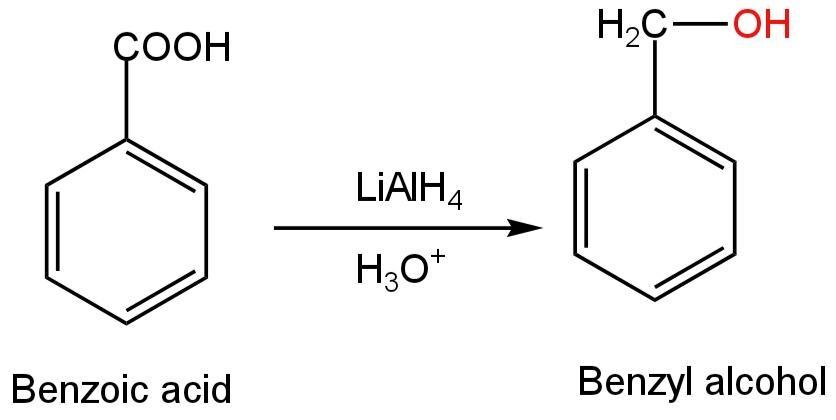
Image: Reduction of benzoic acid.
Thus, benzoic acid can't form phenol or phenoxide ions.
So, B is correct.
C. Benzene diazonium chloride
This compound is formed by the reaction of aniline with sodium nitrite and hydrochloric acid at 273-278 K.
This compound, when warmed with water or dilute acids, undergo hydrolysis to form phenol.
This reaction happens as follows:
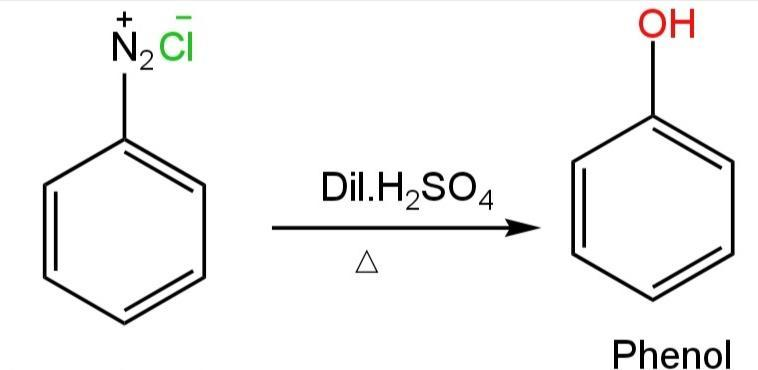
Image: Reaction of benzene diazonium chloride with dilute HCl.
Thus, benzene diazonium chloride forms phenol on treatment with dilute HCl.
So, C is incorrect.
D. Sodium benzenesulfonate
This compound is a sodium salt of benzene sulfonic acid.
This compound if fused with sodium hydroxide at high temperature will form phenol.
The reaction happens as follows:
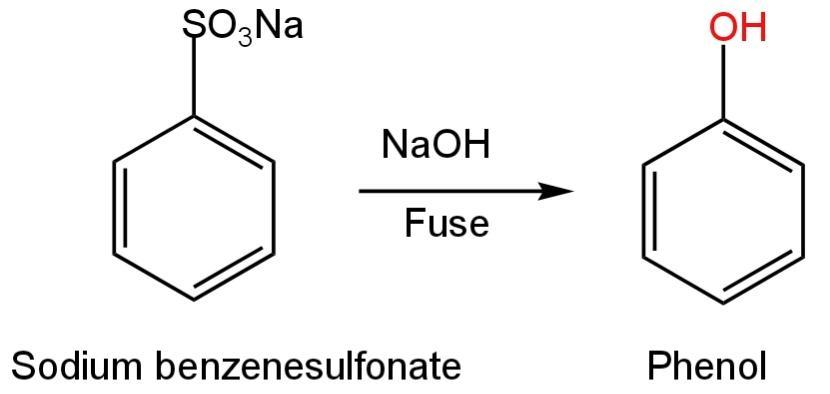
Image: Conversion of sodium benzenesulfonate into phenol.
So, D is incorrect.
Thus, benzoic acid will not form phenol.
So, option B is correct.
Note: The conversion of chlorobenzene into phenol in presence of aqu. NaOH and HCl is known as Dow's process. This is one of the industrial preparation methods of phenol. Another method of preparation of phenol involves the heating of chlorobenzene with superheated steam at 700K in the presence of a catalyst.
Complete Step by Step Answer:
Phenol has the chemical formula \[{C_6}{H_5}OH\].
Phenoxides are salts of phenols.
They may be formed by reaction of phenols with a strong base.
It is also known as phenolates.
In this compound, a benzene ring is attached to an oxygen atom which bears a negative charge due to the loss of the proton.
We have to find out which of the following compounds doesn't form phenol or phenoxide.
A. Chlorobenzene
In this compound, a chlorine atom is attached to the benzene ring.
This compound on reaction with aqueous sodium hydroxide at 623K and at 300 atm pressure forms sodium phenoxide.
Sodium phenoxide reaction with dilute hydrochloric acid forms phenol.
The reaction happens as follows:

Image: Reaction of chlorobenzene with NaOH and HCl.
So, Chlorobenzene forms phenol and phenoxide ions.
So, A is incorrect.
B. Benzoic acid
Benzoic acid in the presence of any reagent or under any drastic conditions will not form phenol or phenoxide ions.
If this compound undergoes reduction, it will form benzyl alcohol not phenol.
The reaction will happen as follows:

Image: Reduction of benzoic acid.
Thus, benzoic acid can't form phenol or phenoxide ions.
So, B is correct.
C. Benzene diazonium chloride
This compound is formed by the reaction of aniline with sodium nitrite and hydrochloric acid at 273-278 K.
This compound, when warmed with water or dilute acids, undergo hydrolysis to form phenol.
This reaction happens as follows:

Image: Reaction of benzene diazonium chloride with dilute HCl.
Thus, benzene diazonium chloride forms phenol on treatment with dilute HCl.
So, C is incorrect.
D. Sodium benzenesulfonate
This compound is a sodium salt of benzene sulfonic acid.
This compound if fused with sodium hydroxide at high temperature will form phenol.
The reaction happens as follows:

Image: Conversion of sodium benzenesulfonate into phenol.
So, D is incorrect.
Thus, benzoic acid will not form phenol.
So, option B is correct.
Note: The conversion of chlorobenzene into phenol in presence of aqu. NaOH and HCl is known as Dow's process. This is one of the industrial preparation methods of phenol. Another method of preparation of phenol involves the heating of chlorobenzene with superheated steam at 700K in the presence of a catalyst.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Difference Between Plant Cell and Animal Cell




