
Which of the following do not exhibit geometrical isomerism (Cis-trans)
(A)
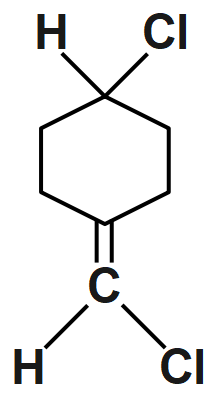
(B) $C{{H}_{3}}CH=CC{{l}_{2}}$
(C)
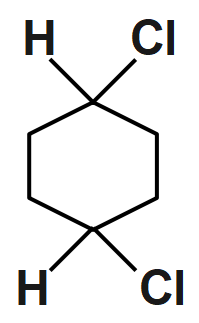
(D)
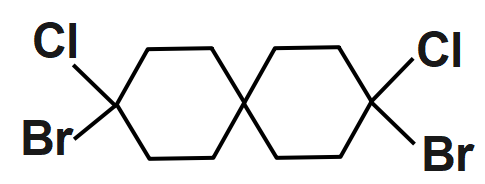


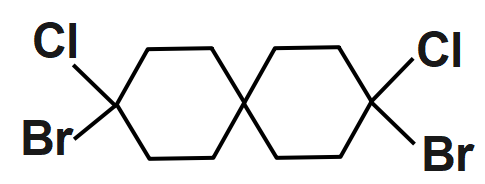
Answer
537.3k+ views
Hint: Those compounds on which same groups are attached to the one carbon atom of the two double bonded carbon atoms don’t show geometrical isomerism or cis-trans isomerism.
Complete step by step solution:
Geometrical isomerism is a kind of configuration isomerism in which different configuration of groups takes place & in other term it is also known as cis or trans isomerism.
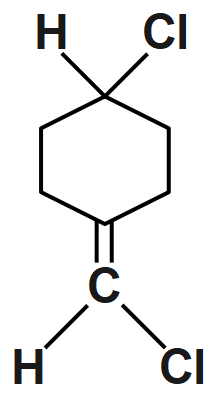
An option (A) compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer.
In option (B) compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer. $C{{H}_{3}}CH=CC{{l}_{2}}$.
An option (C) compound is given and it shows geometrical isomerism because they have different groups attached to the double bonded carbon atoms. Structure of cis & trans isomer of this compound.

An option (D) compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer.
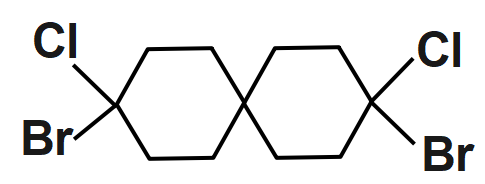
Therefore option A, B and D do not exhibit geometrical isomerism (Cis-trans).
Note:
Here in the option (B) some of you may think that this will not show geometrical isomerism because they have all four groups are different but this is not true because these groups also show cis & trans form on the basis of provided sequence to the attached groups.
Complete step by step solution:
Geometrical isomerism is a kind of configuration isomerism in which different configuration of groups takes place & in other term it is also known as cis or trans isomerism.
An option (A) compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer.

In option (B) compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer. $C{{H}_{3}}CH=CC{{l}_{2}}$.
An option (C) compound is given and it shows geometrical isomerism because they have different groups attached to the double bonded carbon atoms. Structure of cis & trans isomer of this compound.

An option (D) compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer.
Therefore option A, B and D do not exhibit geometrical isomerism (Cis-trans).
Note:
Here in the option (B) some of you may think that this will not show geometrical isomerism because they have all four groups are different but this is not true because these groups also show cis & trans form on the basis of provided sequence to the attached groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




