
Which of the following coordination compounds would exhibit optical isomerism?
A.Pentaamminenitrocobalt\[\left( {III} \right)\] iodide.
B. Diamminedichloroplatinum \[\left( {II} \right)\]
C. Trans-dicyanobis (ethylenediamine) chromium \[\left( {III} \right)\] chloride.
D. Tris-(ethylenediamine) cobalt \[\left( {III} \right)\] bromide.
Answer
492.9k+ views
Hint: Optical isomers are those compounds which have the same number of atoms and connectivity is also the same but they have different spatial arrangement of atoms. Thus we will draw the structure of each compound and then we will analyse its mirror image. If its mirror image is non-superimposable then it is optically active.
Complete answer:
In coordination compounds, the compound which has the same number of atoms but they have different spatial arrangement of atoms then such compounds are known as optical isomers. This property of showing optical isomers is known as optical isomerism. We will draw structure of each compound as:
A.Pentaamminenitrocobalt\[\left( {III} \right)\] iodide
It is represented as \[\left[ {Co{{\left( {N{H_3}} \right)}_5}N{O_2}} \right]{I_2}\]. Its structure can be represented as:
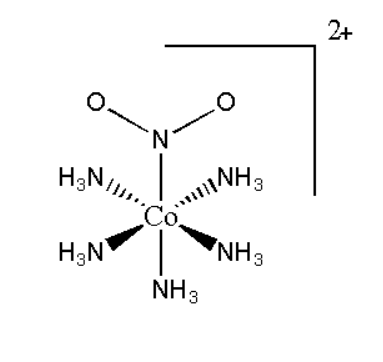
Its mirror image will be superimposable, therefore it will not show optical isomerism.
B. Diamminedichloroplatinum \[\left( {II} \right)\]
Its formula can be represented as \[\left[ {Pt{{\left( {N{H_3}} \right)}_2}C{l_2}} \right]\]. It can be represented as:
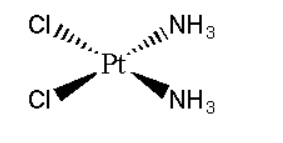
Its mirror image is also superimposable. Hence it does not show optical isomerism.
C. Trans-dicyanobis (ethylenediamine) chromium \[\left( {III} \right)\] chloride
Its molecular formula can be represented as \[\left[ {Cr{{\left( {CN} \right)}_2}{{\left( {en} \right)}_2}} \right]Cl\]
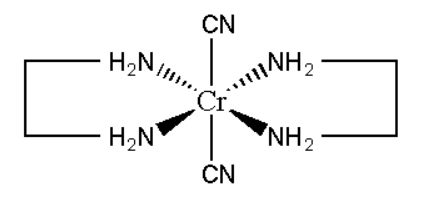
Its mirror image is also superimposable. This does not show optical isomerism.
D. Tris-(ethylenediamine) cobalt \[\left( {III} \right)\] bromide
Its molecular formula can be represented as \[\left[ {Co{{\left( {en} \right)}_2}} \right]Br\]. Its structure can be represented as:
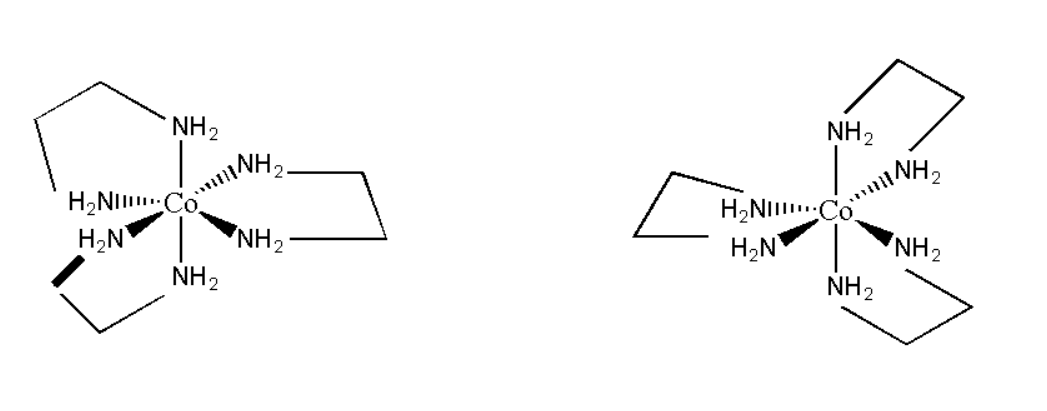
Thus it is asymmetrical in nature and its mirror image is non- superimposable. Hence it will show optical isomerism. Therefore the correct option is D. Tris-(ethylenediamine) cobalt \[\left( {III} \right)\] bromide
Note:
We have neglected the bromide ion in the last part as it is not necessary to study the structure of the compound. The compounds which have unsymmetrical structure generally show optical isomerism. Also these have non- super imposable mirror images because of no symmetry in them. Thus they are optically active in nature.
Complete answer:
In coordination compounds, the compound which has the same number of atoms but they have different spatial arrangement of atoms then such compounds are known as optical isomers. This property of showing optical isomers is known as optical isomerism. We will draw structure of each compound as:
A.Pentaamminenitrocobalt\[\left( {III} \right)\] iodide
It is represented as \[\left[ {Co{{\left( {N{H_3}} \right)}_5}N{O_2}} \right]{I_2}\]. Its structure can be represented as:

Its mirror image will be superimposable, therefore it will not show optical isomerism.
B. Diamminedichloroplatinum \[\left( {II} \right)\]
Its formula can be represented as \[\left[ {Pt{{\left( {N{H_3}} \right)}_2}C{l_2}} \right]\]. It can be represented as:

Its mirror image is also superimposable. Hence it does not show optical isomerism.
C. Trans-dicyanobis (ethylenediamine) chromium \[\left( {III} \right)\] chloride
Its molecular formula can be represented as \[\left[ {Cr{{\left( {CN} \right)}_2}{{\left( {en} \right)}_2}} \right]Cl\]

Its mirror image is also superimposable. This does not show optical isomerism.
D. Tris-(ethylenediamine) cobalt \[\left( {III} \right)\] bromide
Its molecular formula can be represented as \[\left[ {Co{{\left( {en} \right)}_2}} \right]Br\]. Its structure can be represented as:

Thus it is asymmetrical in nature and its mirror image is non- superimposable. Hence it will show optical isomerism. Therefore the correct option is D. Tris-(ethylenediamine) cobalt \[\left( {III} \right)\] bromide
Note:
We have neglected the bromide ion in the last part as it is not necessary to study the structure of the compound. The compounds which have unsymmetrical structure generally show optical isomerism. Also these have non- super imposable mirror images because of no symmetry in them. Thus they are optically active in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




