
Which of the following contains coordinate covalent bonds?
A. \[{{\text{N}}_2}{{\text{H}}_5}^ + \]
B. ${\text{BaC}}{{\text{l}}_2}$
C. ${\text{HCl}}$
D. ${{\text{H}}_2}{\text{O}}$
Answer
589.2k+ views
Hint: Coordinate covalent bond is also known as dative covalent bond. This is a special type of covalent bond in which both the shared electrons are contributed by one atom only. Metal and non-metal usually have ionic bonds. Non-metal and nonmetal bonds with covalent or coordinate bonds.
Complete step by step answer:
Covalent bond is the bond formed by sharing electrons by the atoms. While coordination bond is the
Coordinate covalent bond can be defined as a covalent bond in which both electrons of the shared pair are contributed by one of the two atoms. The atom which donates the electron pair is called donor and the species is called nucleophiles. The atom which accepts them for bond formation is called acceptor and the species is called electrophiles.
Coordinate covalent bond is generally represented by an arrow.
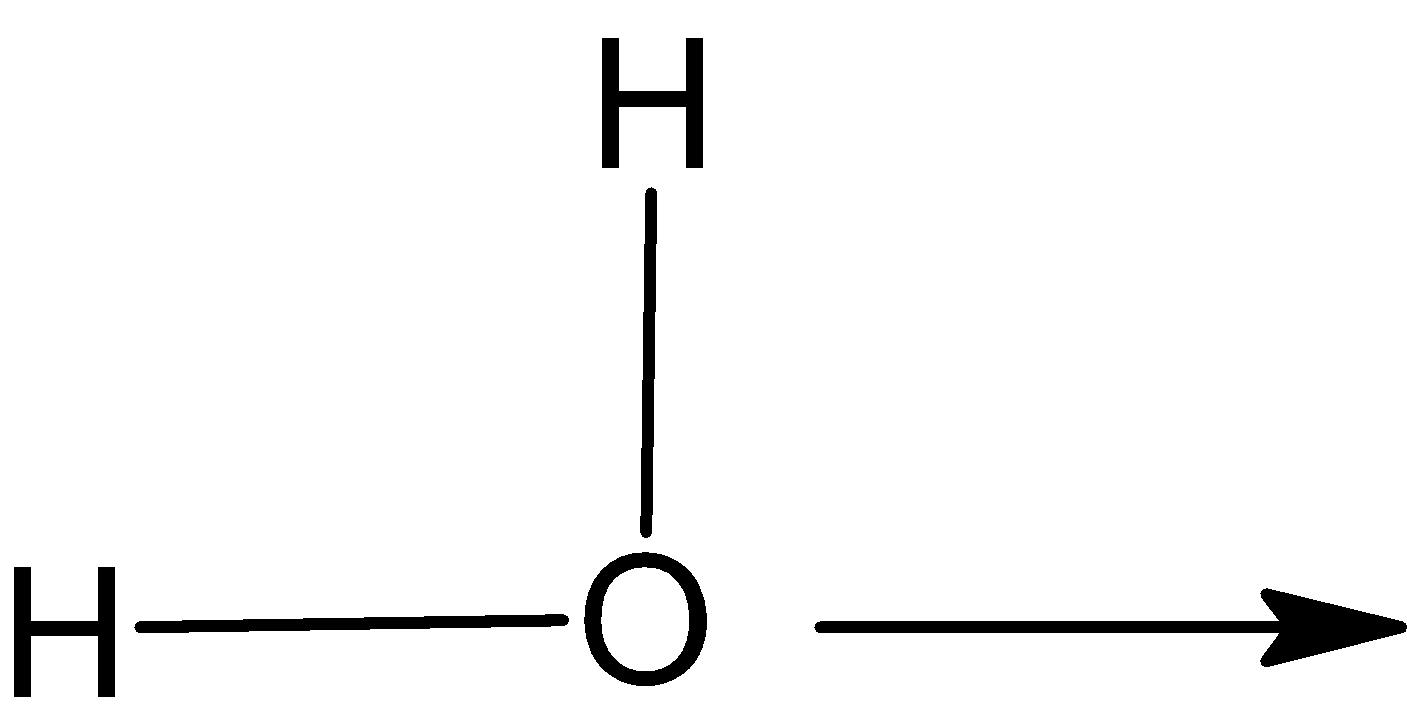 ${\text{H}}$
${\text{H}}$
In this hydronium ion ${{\text{H}}_3}{{\text{O}}^ + }$, head of arrow points toward the acceptor atom. The line is used to represent the covalent bond.
\[{{\text{N}}_2}{{\text{H}}_5}^ + \] molecule is called hydrazinium molecule. It is a combination of ${{\text{N}}_2}{{\text{H}}_4}$ and ${{\text{H}}^ + }$. This is an example for a coordinate covalent bond. Coordinate bonds are formed from nitrogen to hydrogen. Each nitrogen has bonded to three hydrogen.
${\text{BaC}}{{\text{l}}_2}$ is an ionic bond while ${{\text{H}}_2}{\text{O}}$ and ${\text{HCl}}$ are polar covalent bonds.
Hence, Option A is the correct option.
Additional information:
The compound formed via dative covalent bonding can be called an additional compound or adduct. They are very weak bonds. Similar to \[{{\text{N}}_2}{{\text{H}}_5}^ + \], ${{\text{N}}_2}{{\text{O}}_5}$ is also a coordinate covalent bond.
Note:
A coordinate covalent bond is established between two atoms where:
One of the two atoms has a full outer shell and a nonbonding pair of electrons.
The other atom is one pair short of a full outer shell of electrons.
The dative bond once formed is indistinguishable from a regular covalent bond.
Complete step by step answer:
Covalent bond is the bond formed by sharing electrons by the atoms. While coordination bond is the
Coordinate covalent bond can be defined as a covalent bond in which both electrons of the shared pair are contributed by one of the two atoms. The atom which donates the electron pair is called donor and the species is called nucleophiles. The atom which accepts them for bond formation is called acceptor and the species is called electrophiles.
Coordinate covalent bond is generally represented by an arrow.

In this hydronium ion ${{\text{H}}_3}{{\text{O}}^ + }$, head of arrow points toward the acceptor atom. The line is used to represent the covalent bond.
\[{{\text{N}}_2}{{\text{H}}_5}^ + \] molecule is called hydrazinium molecule. It is a combination of ${{\text{N}}_2}{{\text{H}}_4}$ and ${{\text{H}}^ + }$. This is an example for a coordinate covalent bond. Coordinate bonds are formed from nitrogen to hydrogen. Each nitrogen has bonded to three hydrogen.
${\text{BaC}}{{\text{l}}_2}$ is an ionic bond while ${{\text{H}}_2}{\text{O}}$ and ${\text{HCl}}$ are polar covalent bonds.
Hence, Option A is the correct option.
Additional information:
The compound formed via dative covalent bonding can be called an additional compound or adduct. They are very weak bonds. Similar to \[{{\text{N}}_2}{{\text{H}}_5}^ + \], ${{\text{N}}_2}{{\text{O}}_5}$ is also a coordinate covalent bond.
Note:
A coordinate covalent bond is established between two atoms where:
One of the two atoms has a full outer shell and a nonbonding pair of electrons.
The other atom is one pair short of a full outer shell of electrons.
The dative bond once formed is indistinguishable from a regular covalent bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




