
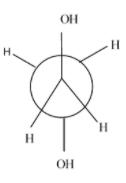
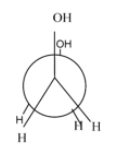
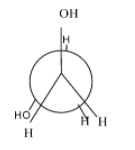
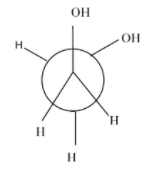
Which of the following conformers for ethylene glycol is most stable?
(A)
(B)
(C)
(D)




Answer
579.6k+ views
Hint: The conformational isomers allow the rotation around the single bond and has four different conformational isomers as anti, eclipsed ,gauche and fully eclipsed and out of this anti is the most stable form not all except in that case if the gauche conformation involves the intermolecular hydrogen bonding. Note identify it.
Complete step by step solution:
First of all, we should know what conformational isomerism is. By the conformational isomers, we mean that the three-dimensional arrangements in space of the atoms in a molecule are interconvertible into one another by the rotation around the single bond. Their interconversion does not require any braking and making of the bonds. This phenomenon is known as conformational isomerism .
In case of ethylene glycol there are four conformational isomers due to the rotation of the atoms around the single bond.
These are;
- Anti-conformation:- it is the most stable form in every conformational isomer and in this all the functional groups and hydrogen atoms are equally placed and there is no repulsion between them.
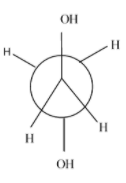
- Eclipsed conformation :- the rotation of anti-conformation at about 60 degrees, it results into eclipsed form and its stability is very always less than the anti-conformation and other conformations.
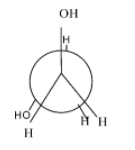
- Gauche conformation:- the rotation of the eclipsed conformation at 60 degrees, results in the gauche conformation. Normally, its second most stable form after the anti but in some cases due to the intermolecular hydrogen bonding ,it is more stable than the anti. Since, in the case of ethylene glycol, it involves intermolecular hydrogen bonding ,it is more stable than the anti-form.
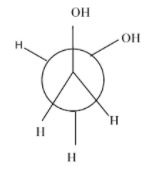
- Fully eclipsed conformation: the rotation of the gauge conformation to 60 degrees results in the gauge conformation and it is the least stable conformation of all.
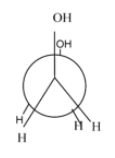
Hence, the most stable conformation of ethylene glycol is gauche conformation.
So, option (D) is correct.
Note: The conformational isomers come under the category of the stereoisomerism which arises due to the difference in the arrangement of atoms in space but resembles one another with respect to which atoms are linked and its study is known as stereochemistry.
Complete step by step solution:
First of all, we should know what conformational isomerism is. By the conformational isomers, we mean that the three-dimensional arrangements in space of the atoms in a molecule are interconvertible into one another by the rotation around the single bond. Their interconversion does not require any braking and making of the bonds. This phenomenon is known as conformational isomerism .
In case of ethylene glycol there are four conformational isomers due to the rotation of the atoms around the single bond.
These are;
- Anti-conformation:- it is the most stable form in every conformational isomer and in this all the functional groups and hydrogen atoms are equally placed and there is no repulsion between them.

- Eclipsed conformation :- the rotation of anti-conformation at about 60 degrees, it results into eclipsed form and its stability is very always less than the anti-conformation and other conformations.

- Gauche conformation:- the rotation of the eclipsed conformation at 60 degrees, results in the gauche conformation. Normally, its second most stable form after the anti but in some cases due to the intermolecular hydrogen bonding ,it is more stable than the anti. Since, in the case of ethylene glycol, it involves intermolecular hydrogen bonding ,it is more stable than the anti-form.

- Fully eclipsed conformation: the rotation of the gauge conformation to 60 degrees results in the gauge conformation and it is the least stable conformation of all.

Hence, the most stable conformation of ethylene glycol is gauche conformation.
So, option (D) is correct.
Note: The conformational isomers come under the category of the stereoisomerism which arises due to the difference in the arrangement of atoms in space but resembles one another with respect to which atoms are linked and its study is known as stereochemistry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




