
Which of the following compounds is optically active?
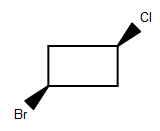
(A)
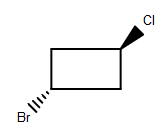
(B)
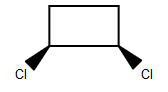
(C)
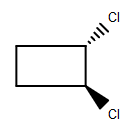
(D)




Answer
583.8k+ views
Hint: For this problem firstly, we have to study the optical and non - optical molecules and also about the chiral and achiral molecules. Then it will become easy to recognise the correct compound which is optically active.
Complete step by step solution:
-In the given question, we have to choose the correct structure which is optically active.
-Firstly, we will study the chiral and achiral molecules. Chiral molecules are those molecules in which the carbon atom is bonded with four different groups whereas if there is any same group attached to the carbon then the molecules are said to be an achiral molecule.
-The properties of chiral and achiral molecules are different, that is the mirror image of the chiral molecules is non-superimposable whereas the mirror image of the achiral molecule is impossible on each other.
-Also, if any molecule consists of chiral carbon but it lacks the symmetry in the molecule such as the centre of molecule and plane of molecule then the molecules are said to be optically active.
-As we can see that among the given structures, all the molecules consist of the plane of symmetry except the structure D.
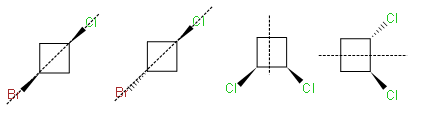
- In structure D, the molecule cannot be divided into equal halves from any angle because one chlorine molecule is up the plane and another chlorine atom is down the plane due to which it is considered as an optically active compound.
Therefore, option (D) is the correct answer.
Note: When a molecule is optically active this indicates that when the ray of plane polarised light is passed through the molecule then it rotates the angle of the plane either in left or in the right direction.
Complete step by step solution:
-In the given question, we have to choose the correct structure which is optically active.
-Firstly, we will study the chiral and achiral molecules. Chiral molecules are those molecules in which the carbon atom is bonded with four different groups whereas if there is any same group attached to the carbon then the molecules are said to be an achiral molecule.
-The properties of chiral and achiral molecules are different, that is the mirror image of the chiral molecules is non-superimposable whereas the mirror image of the achiral molecule is impossible on each other.
-Also, if any molecule consists of chiral carbon but it lacks the symmetry in the molecule such as the centre of molecule and plane of molecule then the molecules are said to be optically active.
-As we can see that among the given structures, all the molecules consist of the plane of symmetry except the structure D.

- In structure D, the molecule cannot be divided into equal halves from any angle because one chlorine molecule is up the plane and another chlorine atom is down the plane due to which it is considered as an optically active compound.
Therefore, option (D) is the correct answer.
Note: When a molecule is optically active this indicates that when the ray of plane polarised light is passed through the molecule then it rotates the angle of the plane either in left or in the right direction.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw the diagram showing the germination of pollen class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




