
Which of the following compounds is optically inactive?
A. 3-Chloro-but-1-ene
B. 2,3- Dichlorobutane
C. 2- Hydroxy propanoic acid
D. 2,2 - Dichloro pentane
Answer
590.4k+ views
Hint: Two kinds of molecules are found i.e. chiral and achiral molecules. The optical property of a molecule depends on the type chirality and achirality of the molecule. The carbon which makes the bond with four different molecules is known as chiral carbon.
Complete Step-by-Step Answer:
-Firstly, we have to identify the chiral and achiral molecule from the given molecules.
-As we know that chiral molecules are those molecules whose mirror images do not superimpose on each other and also they are not symmetrical molecules.
-The molecules also show the optical property because it is observed that when a light beam is passed through the molecule then it starts to rotate in a particular direction.
-There are two directions in which they can move that are clockwise or dextrorotatory and anticlockwise or levorotatory.
-Whereas when a light beam is passed through the achiral molecules then it doesn't rotate in any direction due to which it is considered as an optically inactive molecule.
-So, we have to observe the achiral molecule that is:
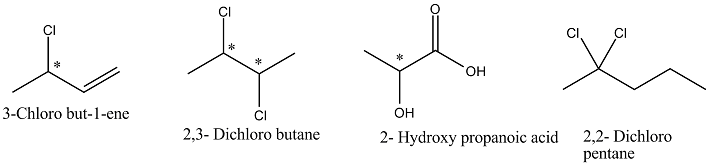
-Above given diagrams is the structure of the molecules given in the question.
-As we can see that all the molecules consist of chiral carbon except 2,2- Dichloro pentane.
-So, we can say that 2,2- Dichloro pentane is achiral molecule due to which it is optically inactive and their mirror image is also superimposable on each other i.e.
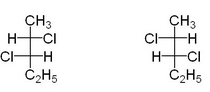
Therefore, option D is the correct answer.
Note: The chiral carbon is also represented by the asterisk ($*$). Racemization is the process in which the optically active solution is converted into the optically inactive solution by the conversion of either leavo into dextro or dextro into leavo direction.
Complete Step-by-Step Answer:
-Firstly, we have to identify the chiral and achiral molecule from the given molecules.
-As we know that chiral molecules are those molecules whose mirror images do not superimpose on each other and also they are not symmetrical molecules.
-The molecules also show the optical property because it is observed that when a light beam is passed through the molecule then it starts to rotate in a particular direction.
-There are two directions in which they can move that are clockwise or dextrorotatory and anticlockwise or levorotatory.
-Whereas when a light beam is passed through the achiral molecules then it doesn't rotate in any direction due to which it is considered as an optically inactive molecule.
-So, we have to observe the achiral molecule that is:

-Above given diagrams is the structure of the molecules given in the question.
-As we can see that all the molecules consist of chiral carbon except 2,2- Dichloro pentane.
-So, we can say that 2,2- Dichloro pentane is achiral molecule due to which it is optically inactive and their mirror image is also superimposable on each other i.e.
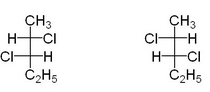
Therefore, option D is the correct answer.
Note: The chiral carbon is also represented by the asterisk ($*$). Racemization is the process in which the optically active solution is converted into the optically inactive solution by the conversion of either leavo into dextro or dextro into leavo direction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




