
Which of the following compounds is most reactive for Wurtz Reaction?
(A) $C{{H}_{3}}CHBrC{{H}_{3}}$
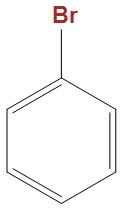
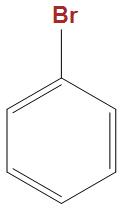
(B)
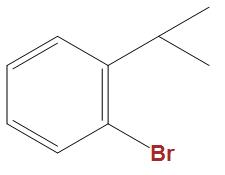
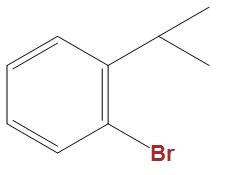
(C)
(D) $C{{H}_{3}}C{{(C{{H}_{3}})}_{2}}Br$


Answer
566.7k+ views
Hint: As we know that Wurtz reaction is a coupling reaction, which is used to prepare symmetrical alkenes. It is basically a reaction in which higher alkanes are synthesised by a reaction in between alkyl halides and metallic sodium. This reaction takes place in presence of dry ether.
Complete answer:
- As we know that less stable radical is more reactive towards Wurtz reaction. We can see the order of reactivity towards Wurtz reaction as:
primary carbocation (least stable) > secondary carbocation (less stable) > tertiary carbocation (more stable)
- We can see that in $C{{H}_{3}}CHBrC{{H}_{3}}$ , there is secondary carbocation present, which is less stable as compared to all other given compounds. Hence, is much reactive towards Wurtz reaction.
- In the option b and c we can see that these are well stabilised by the phenyl ring, hence is not that much reactive towards Wurtz reaction as compared to $C{{H}_{3}}CHBrC{{H}_{3}}$.
- In $C{{H}_{3}}C{{(C{{H}_{3}})}_{2}}Br$ we can see that there is tertiary carbocation present, which is most stable as compared to all other given compounds. Hence, it is not that much reactive towards Wurtz reaction as compared to other given options.
- Hence, we can conclude that the correct option is (a), that is $C{{H}_{3}}CHBrC{{H}_{3}}$ most reactive for Wurtz Reaction.
Note: - It is found that methane is not prepared by this reaction. The minimum number of carbon atoms required is two which is not applicable in the case of methane. Hence, this means that ethane is the lowest alkane developed through this reaction.
Complete answer:
- As we know that less stable radical is more reactive towards Wurtz reaction. We can see the order of reactivity towards Wurtz reaction as:
primary carbocation (least stable) > secondary carbocation (less stable) > tertiary carbocation (more stable)
- We can see that in $C{{H}_{3}}CHBrC{{H}_{3}}$ , there is secondary carbocation present, which is less stable as compared to all other given compounds. Hence, is much reactive towards Wurtz reaction.
- In the option b and c we can see that these are well stabilised by the phenyl ring, hence is not that much reactive towards Wurtz reaction as compared to $C{{H}_{3}}CHBrC{{H}_{3}}$.


- In $C{{H}_{3}}C{{(C{{H}_{3}})}_{2}}Br$ we can see that there is tertiary carbocation present, which is most stable as compared to all other given compounds. Hence, it is not that much reactive towards Wurtz reaction as compared to other given options.
- Hence, we can conclude that the correct option is (a), that is $C{{H}_{3}}CHBrC{{H}_{3}}$ most reactive for Wurtz Reaction.
Note: - It is found that methane is not prepared by this reaction. The minimum number of carbon atoms required is two which is not applicable in the case of methane. Hence, this means that ethane is the lowest alkane developed through this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




