
Which of the following compounds have higher enolic content than keto content?
A.
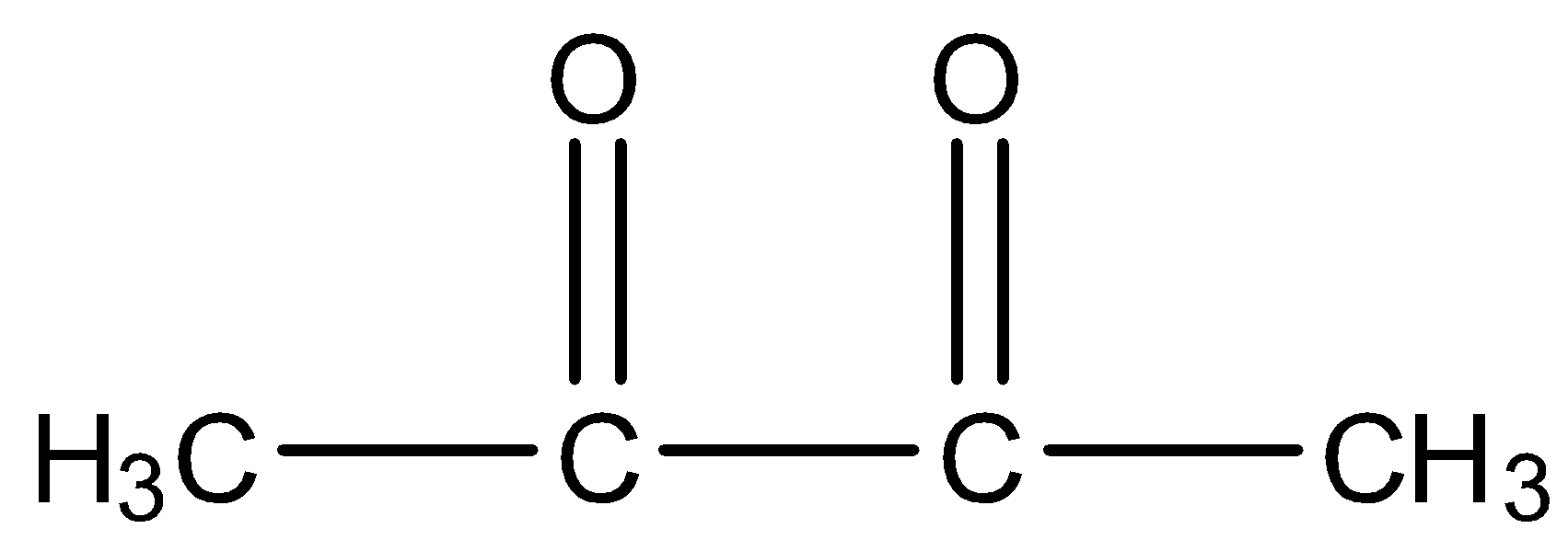
B.
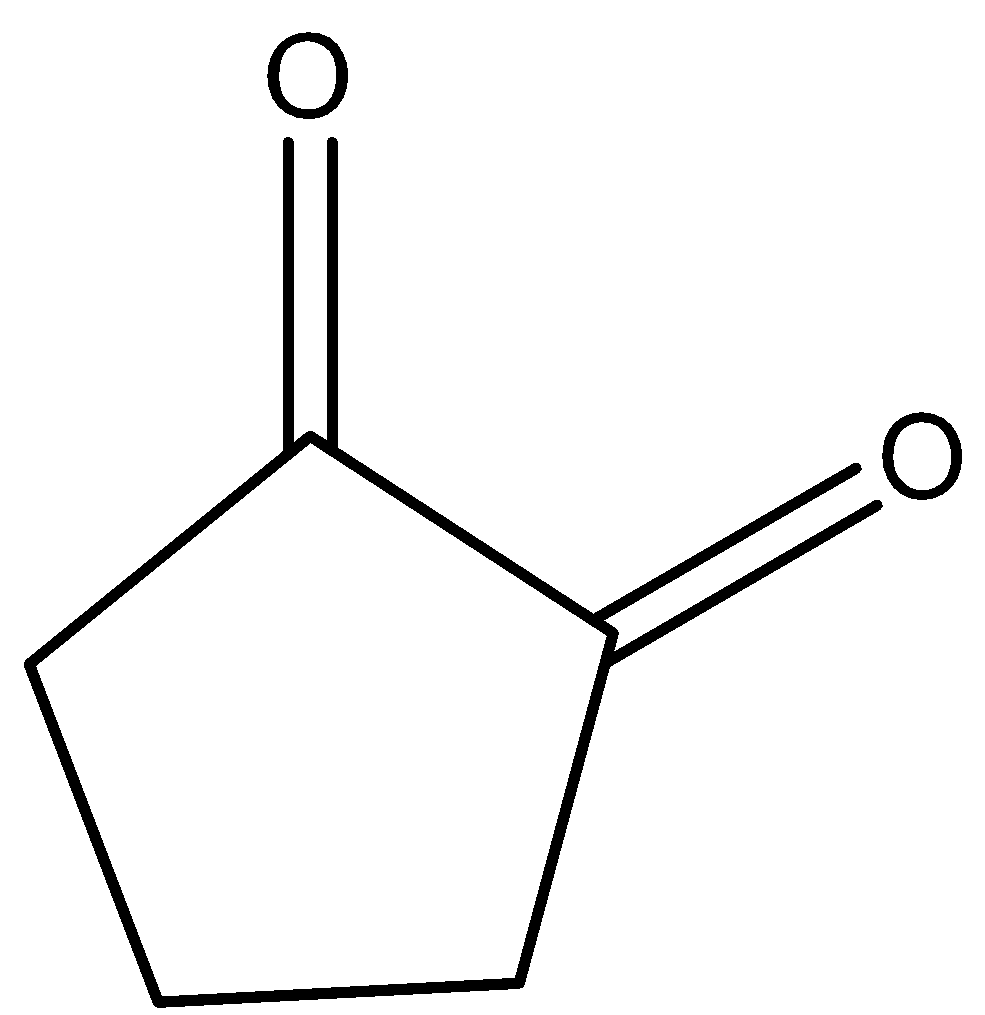
C.
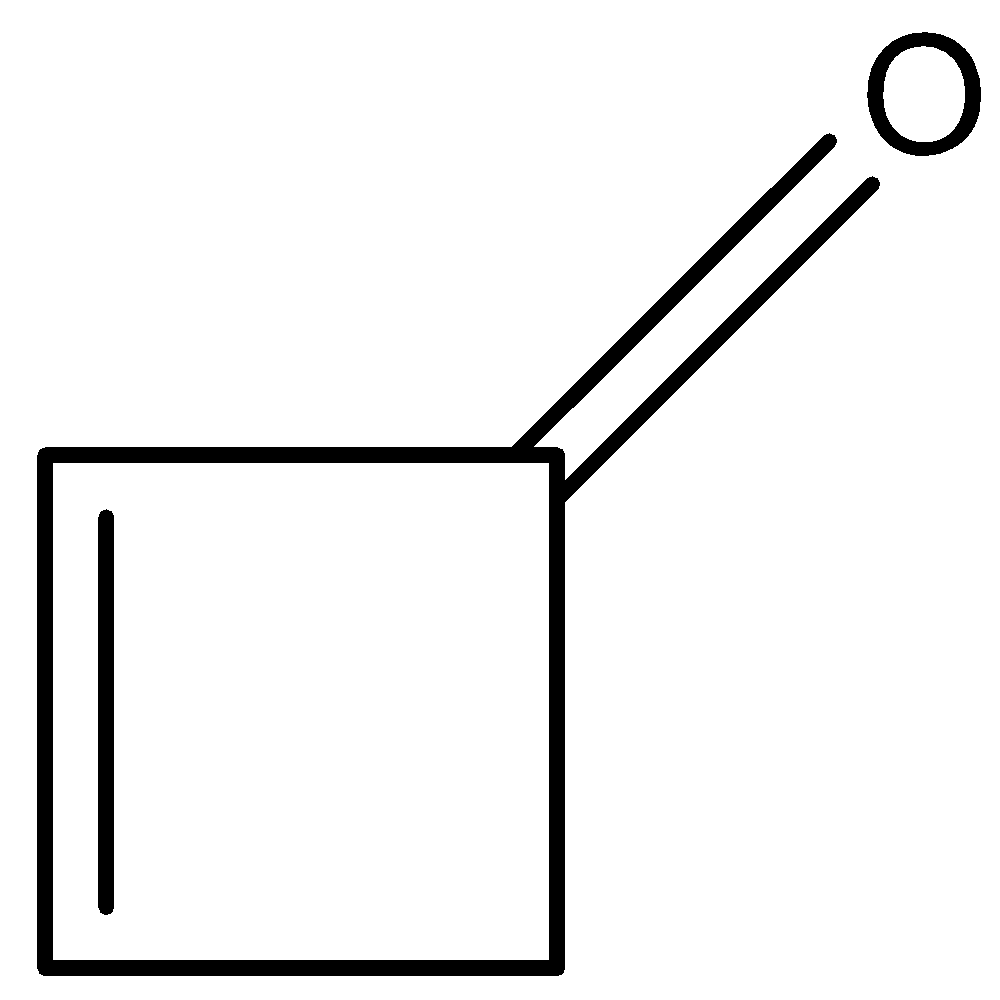
D.
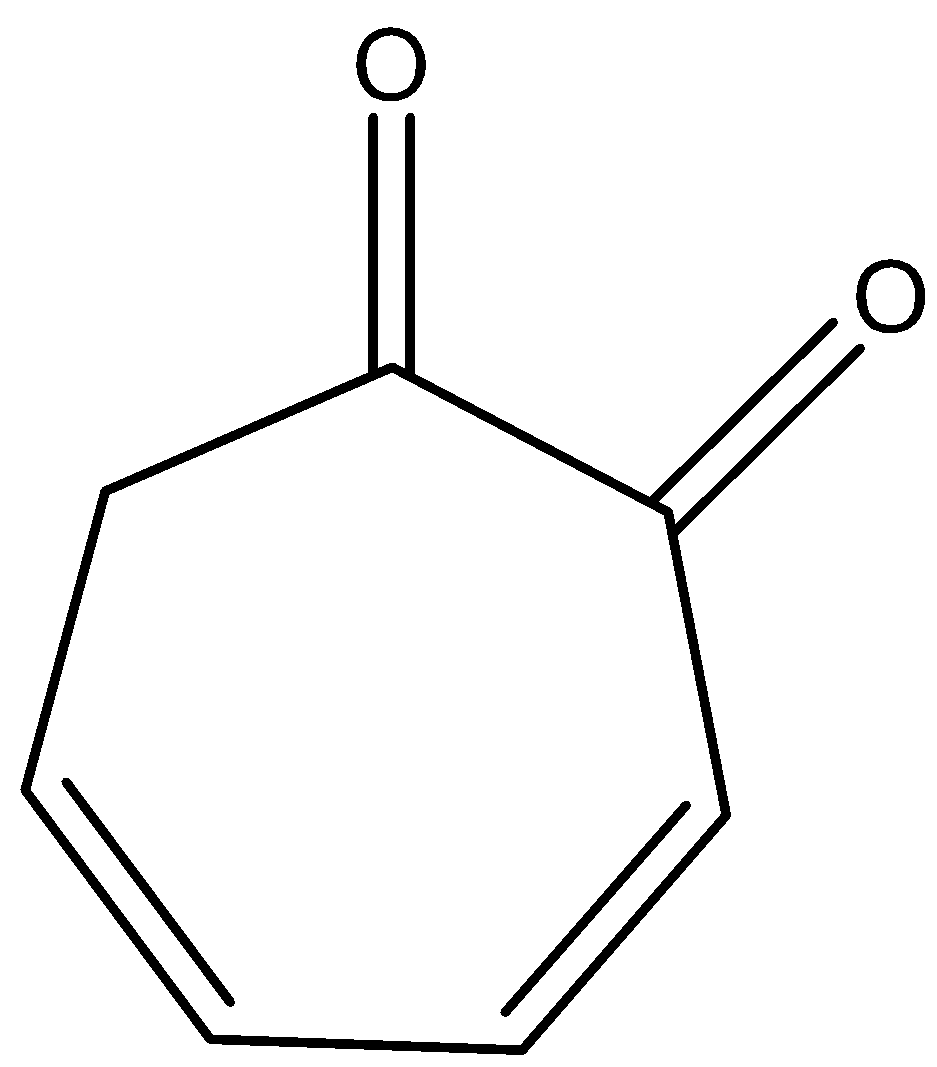




Answer
570k+ views
Hint: Generally the ketone compounds exhibit the conversion of keto form to enol form through keto-enol tautomerism. To exhibit keto-enol tautomerism the carbonyl compound should contain alpha hydrogen atoms in it.
Complete Solution :
- In the question it is given that which compound shows high enolic content when compared to keto form among the given options.
- In the given options all the compounds exhibit keto-enol tautomerism but we have to find which compound will show high enoilc content when compared to keto form.
- The keto-enol tautomerism of all the given options is as follows.
- The keto-enol tautomerism Option A is as follows.
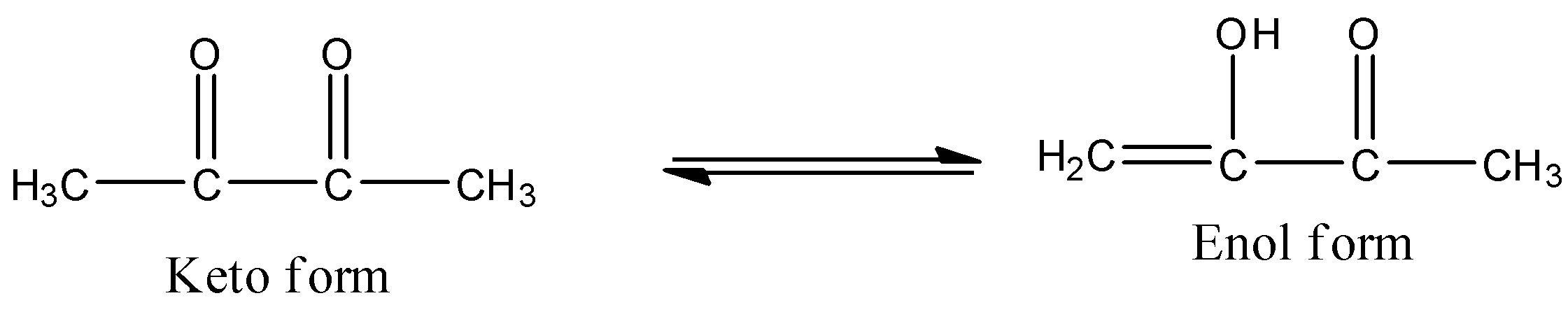
- The keto-enol tautomerism Option B is as follows.
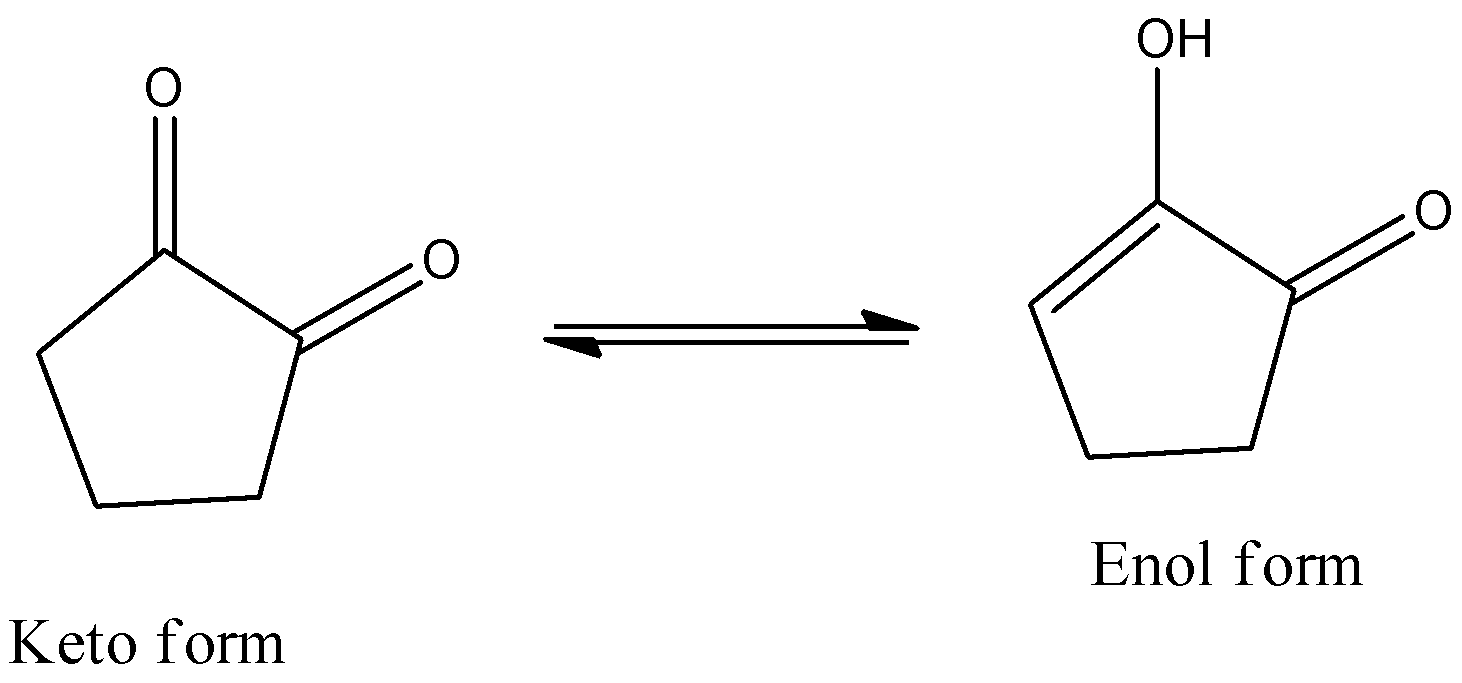
- The keto-enol tautomerism Option C is as follows.
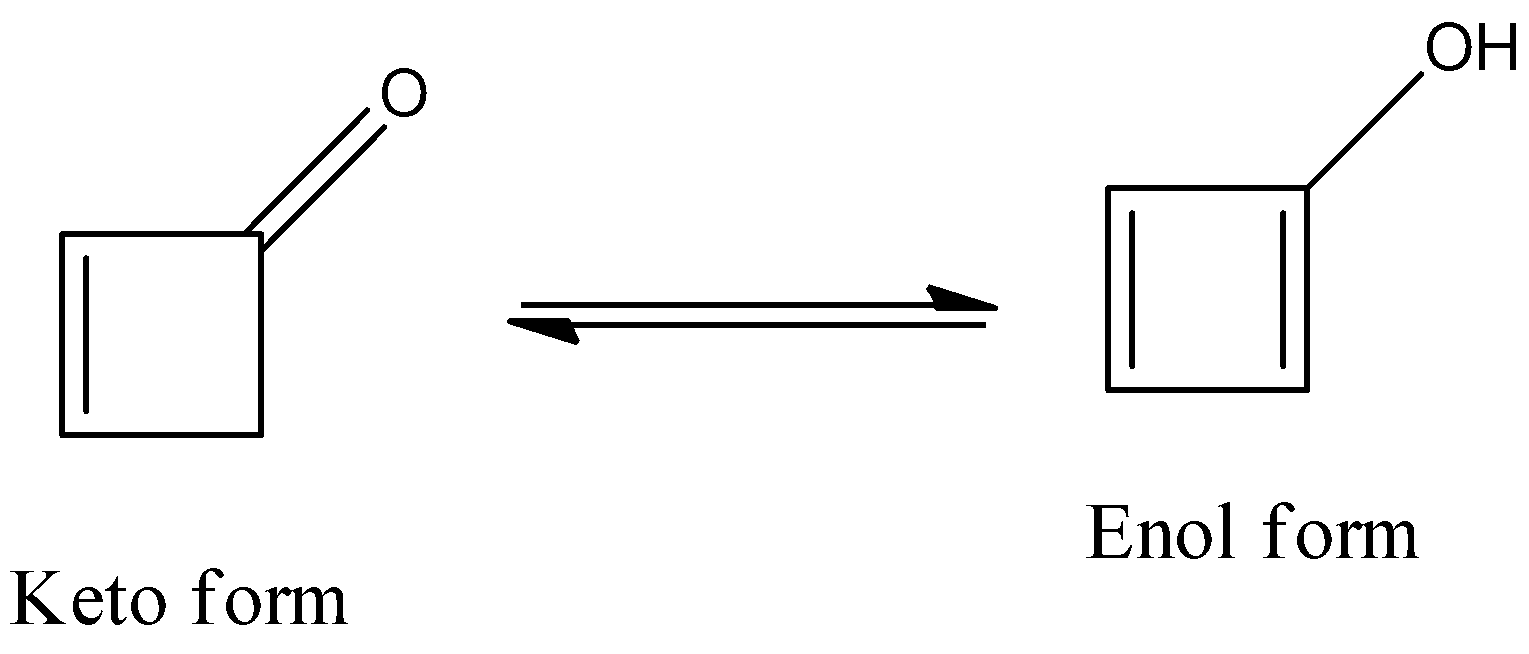
- The keto-enol tautomerism Option D is as follows.
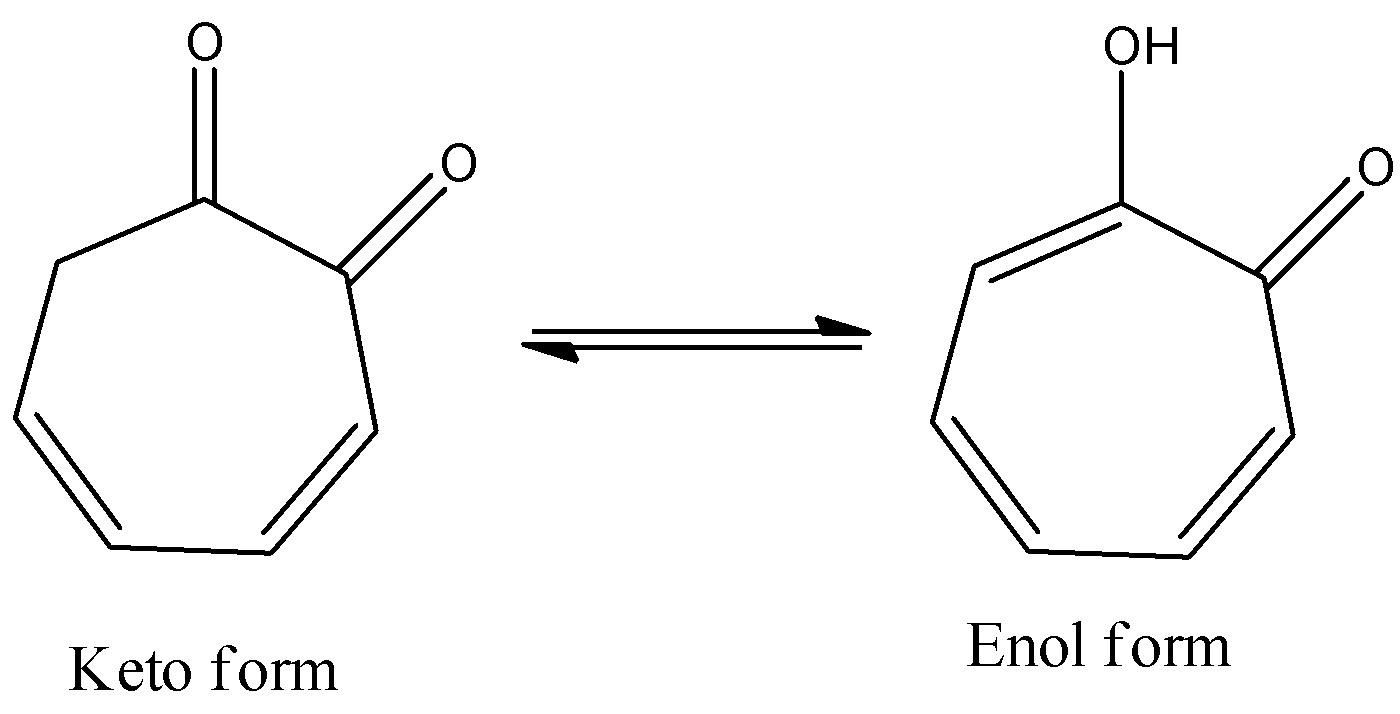
- The option B will show high enolic content due to the presence of hydrogen bond between the alcohol and ketone.
- The presence of hydrogen bond will make high enolic content in the compound B and it is as follows.
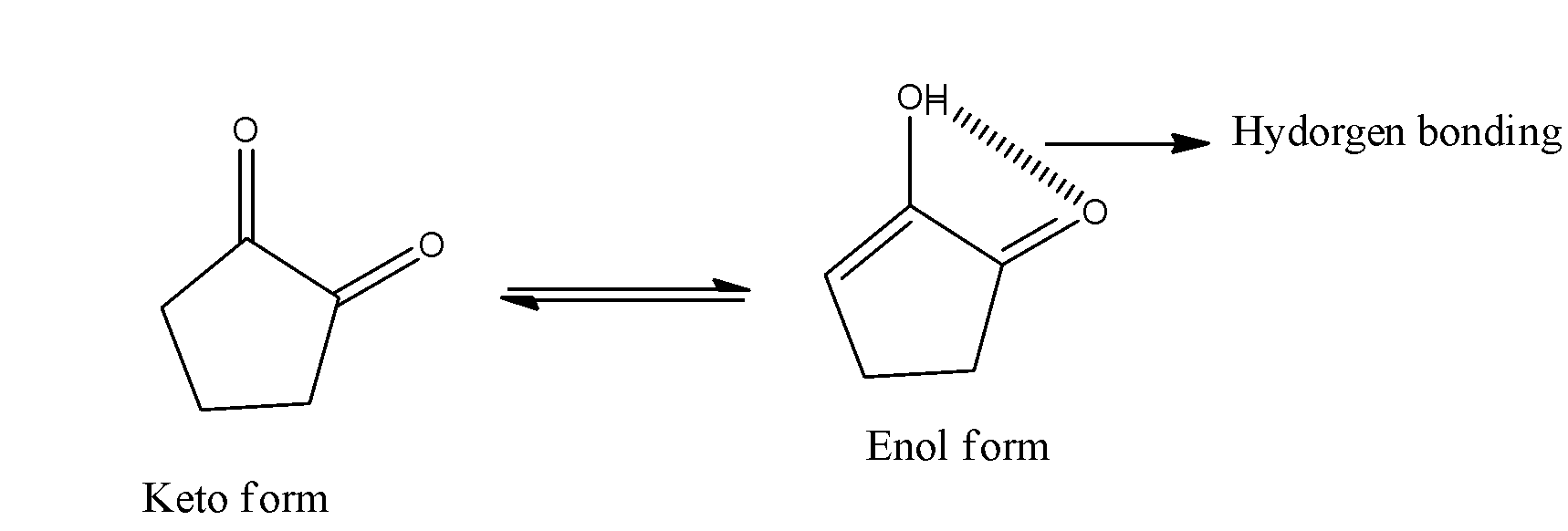
- The presence of the hydrogen bonding is the reason behind the high enolic content in option C.
So, the correct answer is “Option C”.
Note: Generally the keto form is more stable at room temperature. But few compounds will exhibit high enolic content at room temperature due to the presence of intramolecular hydrogen bonding means the hydrogen bond is going to form internally in the molecule itself.
Complete Solution :
- In the question it is given that which compound shows high enolic content when compared to keto form among the given options.
- In the given options all the compounds exhibit keto-enol tautomerism but we have to find which compound will show high enoilc content when compared to keto form.
- The keto-enol tautomerism of all the given options is as follows.
- The keto-enol tautomerism Option A is as follows.

- The keto-enol tautomerism Option B is as follows.

- The keto-enol tautomerism Option C is as follows.

- The keto-enol tautomerism Option D is as follows.

- The option B will show high enolic content due to the presence of hydrogen bond between the alcohol and ketone.
- The presence of hydrogen bond will make high enolic content in the compound B and it is as follows.

- The presence of the hydrogen bonding is the reason behind the high enolic content in option C.
So, the correct answer is “Option C”.
Note: Generally the keto form is more stable at room temperature. But few compounds will exhibit high enolic content at room temperature due to the presence of intramolecular hydrogen bonding means the hydrogen bond is going to form internally in the molecule itself.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




