
Which of the following compounds has a zero dipole moment?
A. 1. 1 Dichloroethylene
B. Cis 1.2 dichloroethylene
C. Trans-1,2—dichloroethylene
D. Both 2 and 3
Answer
588.6k+ views
Hint: Dipole moment is a quantity that describes two charges that are separated by a distance. It is a quantity that we can measure the quantity in the lab for a molecule to determine the bond distance, size of the partial charges of the molecules. The dipole moment is also measuring the polarity of the molecule.
Complete step by step answer:
The structure of and Cis 1.2 dichloroethylene is shown below,
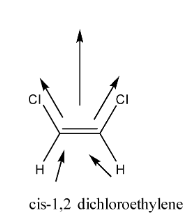
In the given structure it is clear that C is 1.2 dichloroethylene shows dipole moment. As chlorine is more electronegative than hydrogen, the overall dipole moment is towards chlorine.
On the other hand, structures of Trans-1,2—dichloroethylene and 1. 1 Dichloro ethylene is shown below.
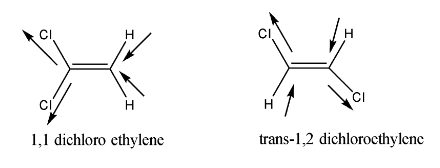
From the given structures it is clear that Trans-1,2—dichloroethylene and 1. 1 Dichloroethylene has no dipole moment, as the bond dipoles of each structure get nullified with each other.
Note: A molecule is called to be a nonpolar molecule if there is an equivalent sharing of electrons between two atoms of a molecule which is diatomic in nature, or if there is symmetry in the polar bonds of a significantly complex molecule. As well as when the dipole moment is zero of a molecule then it is called a nonpolar molecule. And if there is a dipole moment then it is called a polar molecule.
Complete step by step answer:
The structure of and Cis 1.2 dichloroethylene is shown below,

In the given structure it is clear that C is 1.2 dichloroethylene shows dipole moment. As chlorine is more electronegative than hydrogen, the overall dipole moment is towards chlorine.
On the other hand, structures of Trans-1,2—dichloroethylene and 1. 1 Dichloro ethylene is shown below.

From the given structures it is clear that Trans-1,2—dichloroethylene and 1. 1 Dichloroethylene has no dipole moment, as the bond dipoles of each structure get nullified with each other.
Note: A molecule is called to be a nonpolar molecule if there is an equivalent sharing of electrons between two atoms of a molecule which is diatomic in nature, or if there is symmetry in the polar bonds of a significantly complex molecule. As well as when the dipole moment is zero of a molecule then it is called a nonpolar molecule. And if there is a dipole moment then it is called a polar molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




