
Which of the following compounds does not contain a carbonyl group?
(A). Acetylacetone
(B). Diethyl malonate
(C). Amines
(D). Meldrum’s acid
Answer
505.8k+ views
Hint: Organic chemistry is the branch of the chemistry that deals with the study of the hydrocarbons and several other functional groups like, halogens, alcohols, phenol, ethers, aldehydes, ketones, carboxylic acids, have nitrogen, oxygen, and other atoms as functional groups. Each functional group has a separate identity and different chemical and physical properties.
Complete answer:
Organic Chemistry has several functional groups like halogens, alcohols, phenol, ethers, aldehydes, ketones, carboxylic acids, nitrogen, oxygen, and other atoms. However, the collective group of aldehydes, ketones and carboxylic acids is known as the Carbonyl group. The common thing in all is the presence of $ {(R)_2} - C = O $ . In case of aldehydes, one of the R is hydrogen represented as $ R - CH = O $ in case of ketones both R may be same or different having representation as $ {(R)_2} - C = O $ or $ R - C{R'} = O $ in case of carboxylic acids the general representation is as $ R - COOH $ .
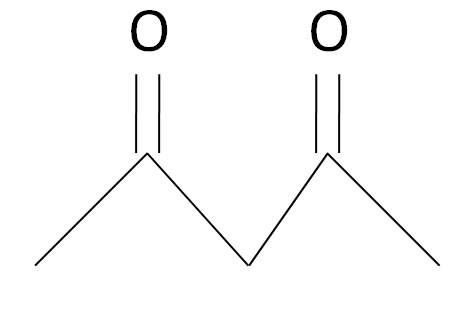
The acetylacetone is a carbonyl complex which has a structure as given: -
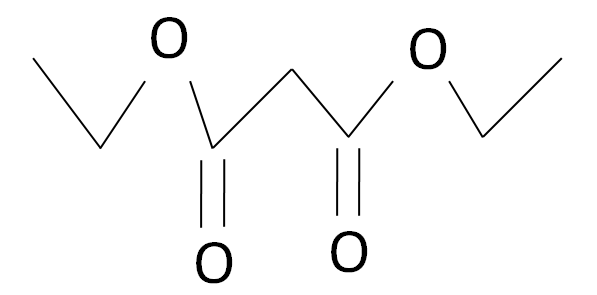
The Diethyl malonate is a carbonyl complex which has a structure as given: -
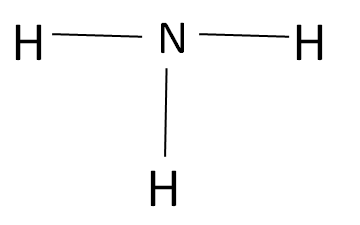
The Amine is an organic compound which does not contain any type of carbonyl group as shown: -
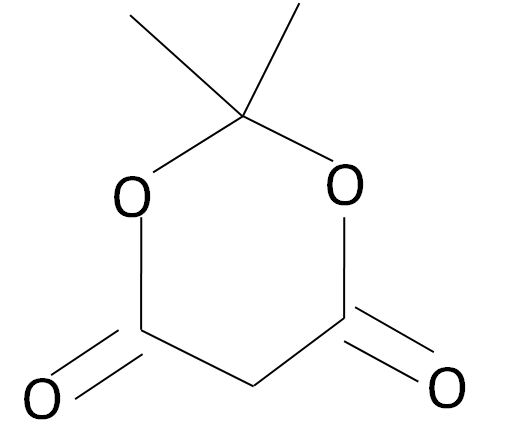
The Meldrum’s acid is a carbonyl complex which has a structured as given: -
Note:
The carbonyl group is a special group in organic chemistry which consists of three different types of functional groups namely aldehydes, ketones and carboxylic acids. According to the I.U.P.A.C (International Union of Pure and Applied Chemistry) the suffixes of their names are –al for aldehydes, -One for ketones and –Oic acids for carboxylic acids.
Complete answer:
Organic Chemistry has several functional groups like halogens, alcohols, phenol, ethers, aldehydes, ketones, carboxylic acids, nitrogen, oxygen, and other atoms. However, the collective group of aldehydes, ketones and carboxylic acids is known as the Carbonyl group. The common thing in all is the presence of $ {(R)_2} - C = O $ . In case of aldehydes, one of the R is hydrogen represented as $ R - CH = O $ in case of ketones both R may be same or different having representation as $ {(R)_2} - C = O $ or $ R - C{R'} = O $ in case of carboxylic acids the general representation is as $ R - COOH $ .
The acetylacetone is a carbonyl complex which has a structure as given: -

The Diethyl malonate is a carbonyl complex which has a structure as given: -

The Amine is an organic compound which does not contain any type of carbonyl group as shown: -

The Meldrum’s acid is a carbonyl complex which has a structured as given: -

Note:
The carbonyl group is a special group in organic chemistry which consists of three different types of functional groups namely aldehydes, ketones and carboxylic acids. According to the I.U.P.A.C (International Union of Pure and Applied Chemistry) the suffixes of their names are –al for aldehydes, -One for ketones and –Oic acids for carboxylic acids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




