
Which of the following cannot exhibit stereo-isomerism.
(A)-${\text{C}}{{\text{H}}_{\text{3}}}{\text{CH}}\left( {{\text{Cl}}} \right){\text{COOH}}$
(B)-${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CH = NOH}}$
(C)-${\text{ClCH = CHCl}}$
(D)-${\text{ClCH = C}}{{\text{H}}_{\text{2}}}$
Answer
566.7k+ views
Hint: Stereoisomers show by those isomers which have the same molecular formula but showing different structures due to different arrangements of atoms or bonds in the space of the given molecule. Those molecules which show optical activity or geometrical isomerism also come under stereo-isomers.
Complete answer:
-In the option (A), compound ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CH}}\left( {{\text{Cl}}} \right){\text{COOH}}$ is given which is showing optical activity or optical isomerism due to the presence of chiral carbon center in it.
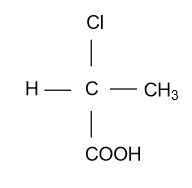
In the above figure, optical activity is shown due to the center carbon because all 4 vacancies are fulfilled by 4 different groups that’s why it is chiral carbon.
-In the option (B), compound ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CH = NOH}}$ is given which is showing geometrical isomerism due to the presence of double bond and different arrangements of substituents present on carbon and nitrogen atom attached to double bond.
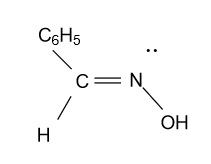
In the above figure a lone pair present on a nitrogen atom behaves as a substituent group on nitrogen and showing geometrical isomerism.
-In the option (C), compound ${\text{ClCH = CHCl}}$ is given which is showing geometrical isomerism due to the presence of double bond, 2 different groups on each carbon atom and different arrangements of substituents.
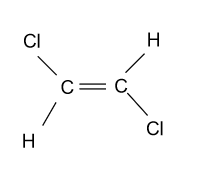
-In the option (D), compound ${\text{ClCH = C}}{{\text{H}}_{\text{2}}}$ is given which is not showing stereo isomerism due to the absence of chiral carbon and presence of 2 same group i.e. hydrogen on one carbon atom.
Hence, option (D) i.e. ${\text{ClCH = C}}{{\text{H}}_{\text{2}}}$ among all given compounds cannot exhibit stereo-isomerism.
Note: In finding stereoisomers never get confused with the structural isomerism, because there is only a very small difference between structural and stereoisomers. So, always keep in mind that in structural isomers chiral carbon is not present.
Complete answer:
-In the option (A), compound ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CH}}\left( {{\text{Cl}}} \right){\text{COOH}}$ is given which is showing optical activity or optical isomerism due to the presence of chiral carbon center in it.

In the above figure, optical activity is shown due to the center carbon because all 4 vacancies are fulfilled by 4 different groups that’s why it is chiral carbon.
-In the option (B), compound ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{CH = NOH}}$ is given which is showing geometrical isomerism due to the presence of double bond and different arrangements of substituents present on carbon and nitrogen atom attached to double bond.

In the above figure a lone pair present on a nitrogen atom behaves as a substituent group on nitrogen and showing geometrical isomerism.
-In the option (C), compound ${\text{ClCH = CHCl}}$ is given which is showing geometrical isomerism due to the presence of double bond, 2 different groups on each carbon atom and different arrangements of substituents.

-In the option (D), compound ${\text{ClCH = C}}{{\text{H}}_{\text{2}}}$ is given which is not showing stereo isomerism due to the absence of chiral carbon and presence of 2 same group i.e. hydrogen on one carbon atom.
Hence, option (D) i.e. ${\text{ClCH = C}}{{\text{H}}_{\text{2}}}$ among all given compounds cannot exhibit stereo-isomerism.
Note: In finding stereoisomers never get confused with the structural isomerism, because there is only a very small difference between structural and stereoisomers. So, always keep in mind that in structural isomers chiral carbon is not present.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




