
Which of the following can be produced by wurtz reaction in good yield?
(A)
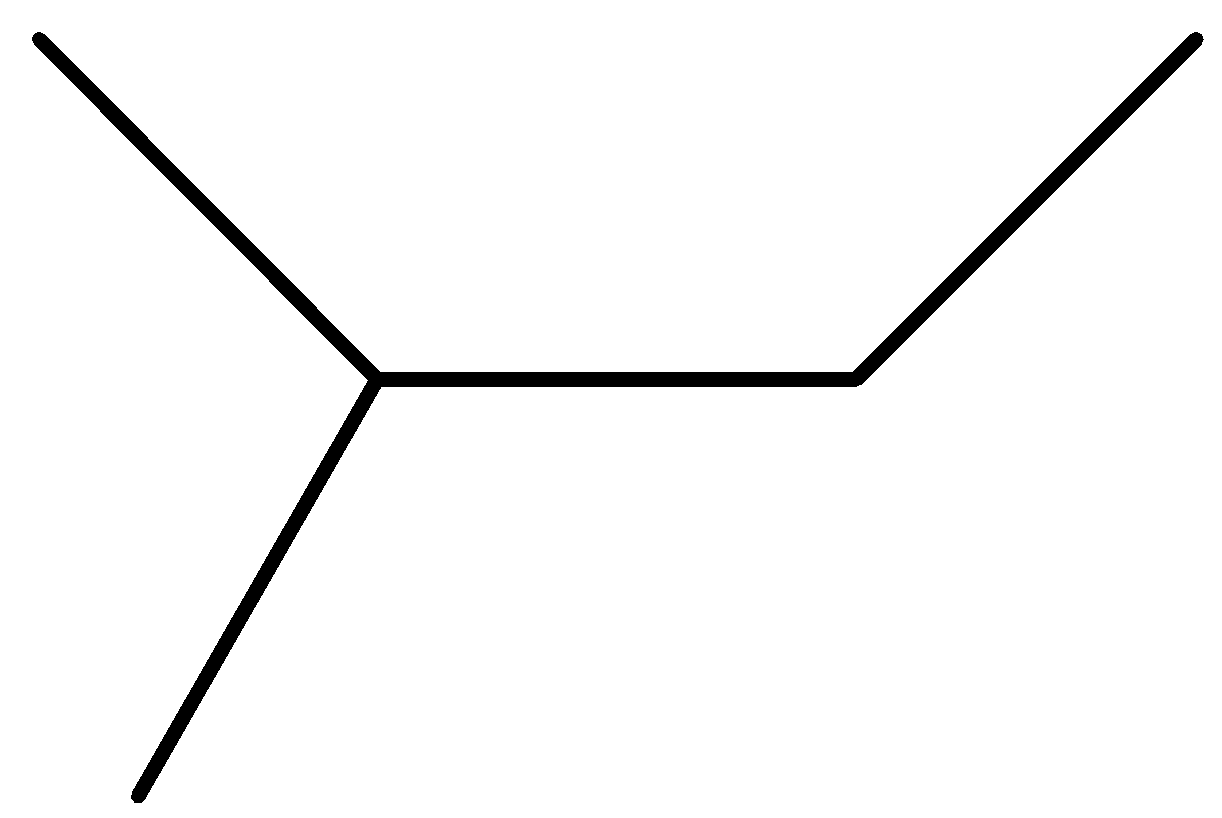
(B)
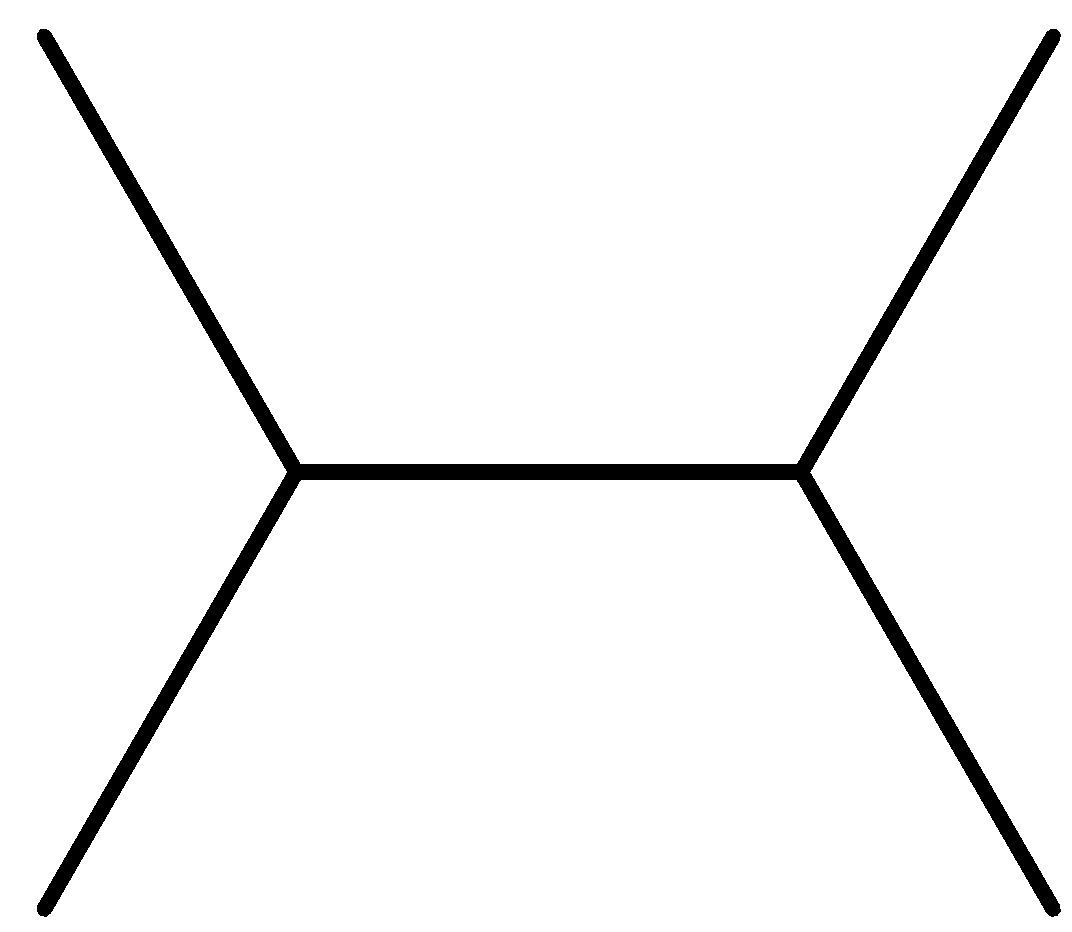
(C)
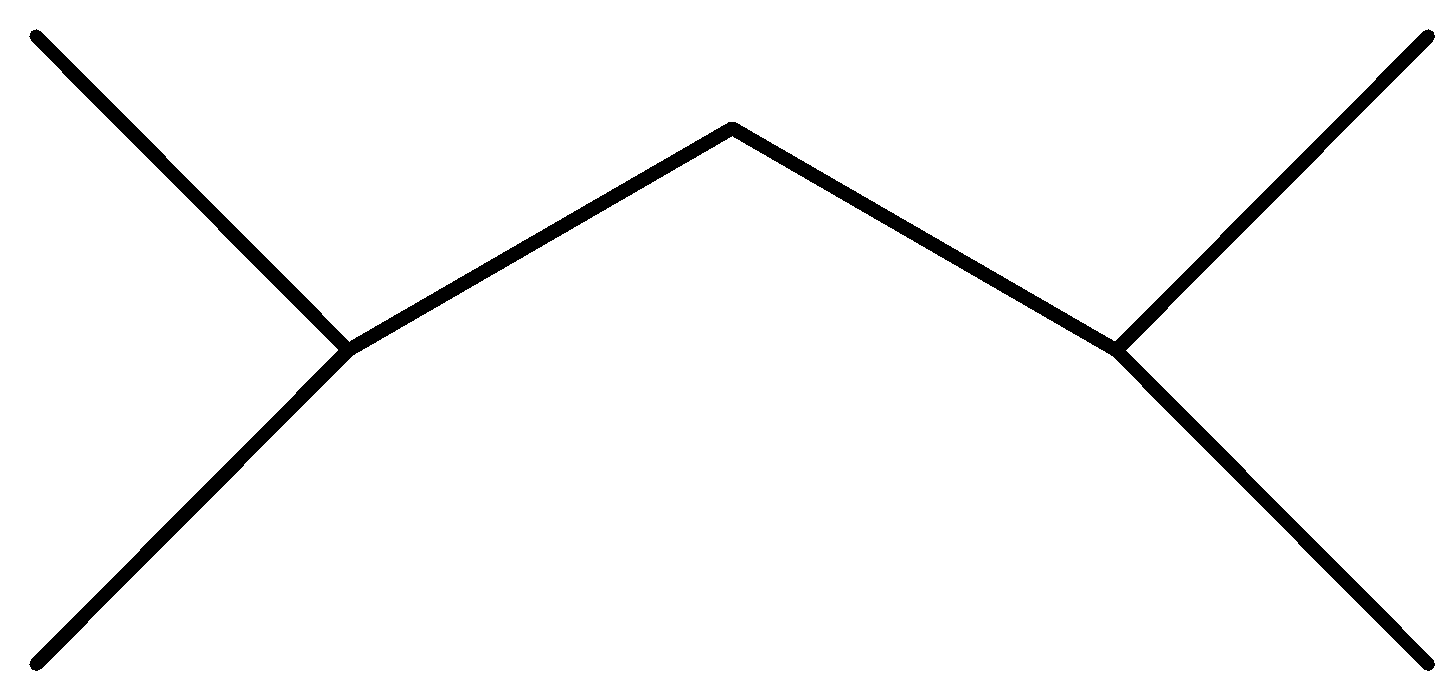
(D)
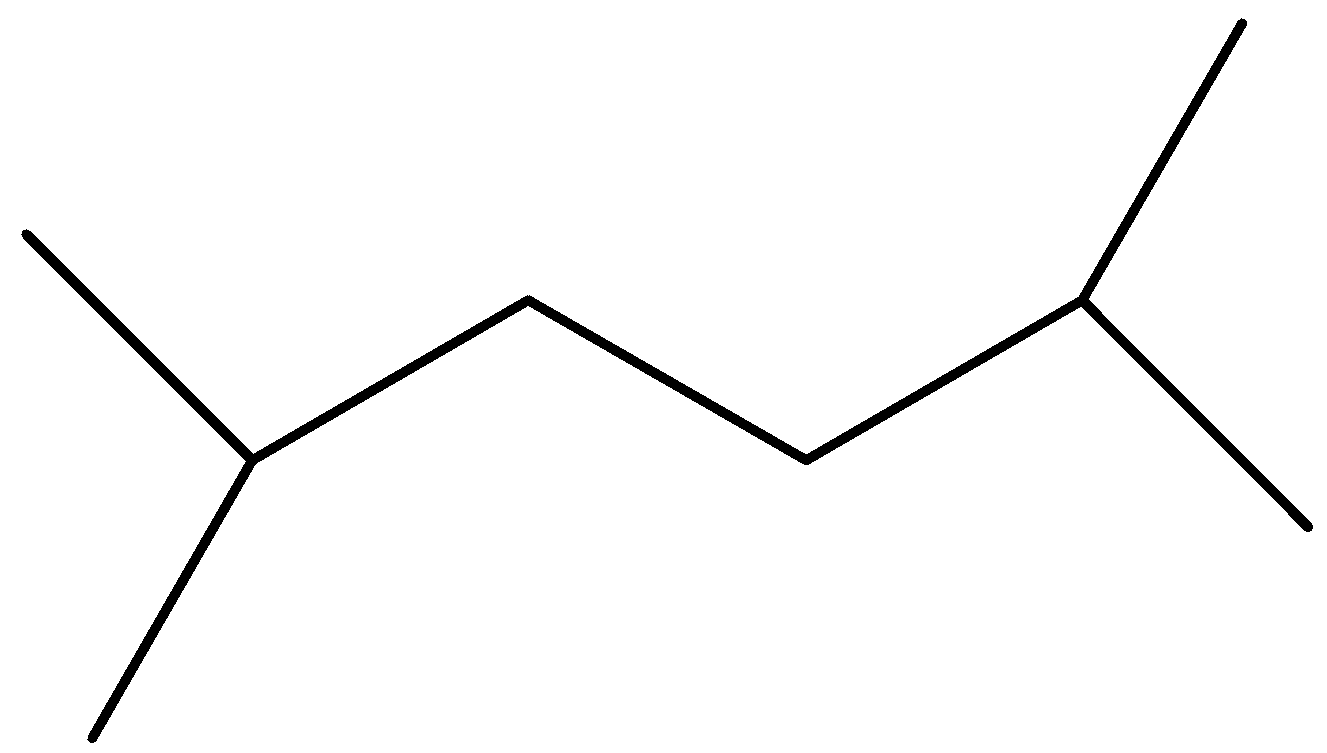




Answer
558.3k+ views
Hint: First we will learn about the mechanism and conditions for a wurtz reaction as to what kind of substrate it acts upon and then we will know what kind of substrate produces a high yield product according to the mechanism.
Complete step by step answer:
Wurtz reaction is a type of coupling reaction. A coupling reaction in which metal is used so it is a type of organometallic reaction. In this reaction two molecules of alkyl halide couple with each other in presence of sodium metal and dry ether to produce a higher alkane. The two molecules of alkyl halide used can be the same or can be different also. But a very good yield will be produced only when the reactants are same and symmetrical.
Mechanism of wurtz reaction is:
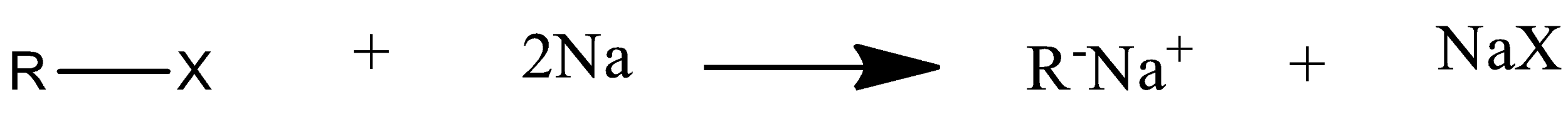
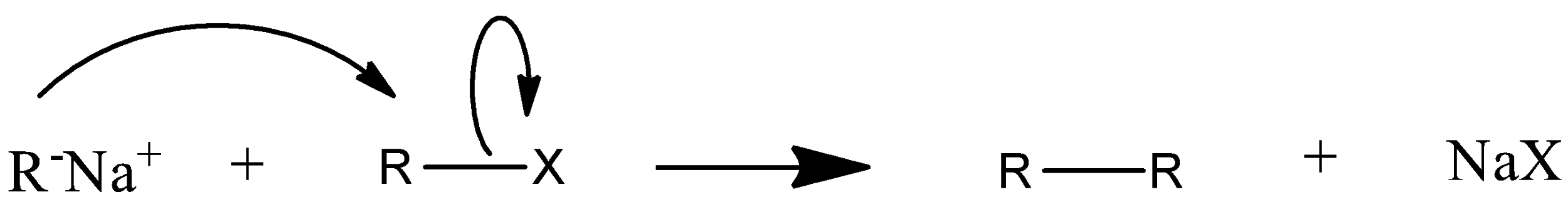
So this is how a higher alkane is formed from wurtz coupling.
Now for good yield the reactants must be symmetric and reactants must be the same.
Option A is not symmetrical so not the correct answer.
Option B is symmetrical as well as the reactant will be similar as ${\text{2 - chloropropane}}$
Option C is not symmetrical and reactants will also not be the same. One will be ${\text{2 - chloropropane}}$ other will be ${\text{1 - chloro - 2 - methylpropane}}$.
Option D is symmetrical but again the reactants are not similar.
So, the correct answer is Option B.
Additional Information:
Just like the wurtz reaction there is a wurtz fittig reaction also which is also a coupling reaction. In wurtz fittig the reactants are aryl halides with alkyl halides so that we can form aromatic products also.
Note: In wurtz coupling reaction it is mandatory to use only dry ether and not moist ether . The reason is that the moist ether contains water molecules and we all are aware that sodium reacts violently with water so the reaction cannot be done in moist condition.
Complete step by step answer:
Wurtz reaction is a type of coupling reaction. A coupling reaction in which metal is used so it is a type of organometallic reaction. In this reaction two molecules of alkyl halide couple with each other in presence of sodium metal and dry ether to produce a higher alkane. The two molecules of alkyl halide used can be the same or can be different also. But a very good yield will be produced only when the reactants are same and symmetrical.
Mechanism of wurtz reaction is:
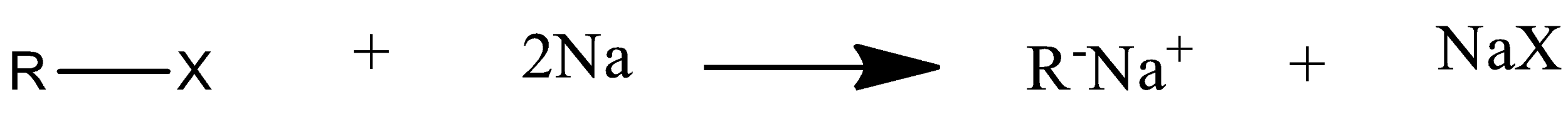

So this is how a higher alkane is formed from wurtz coupling.
Now for good yield the reactants must be symmetric and reactants must be the same.
Option A is not symmetrical so not the correct answer.
Option B is symmetrical as well as the reactant will be similar as ${\text{2 - chloropropane}}$
Option C is not symmetrical and reactants will also not be the same. One will be ${\text{2 - chloropropane}}$ other will be ${\text{1 - chloro - 2 - methylpropane}}$.
Option D is symmetrical but again the reactants are not similar.
So, the correct answer is Option B.
Additional Information:
Just like the wurtz reaction there is a wurtz fittig reaction also which is also a coupling reaction. In wurtz fittig the reactants are aryl halides with alkyl halides so that we can form aromatic products also.
Note: In wurtz coupling reaction it is mandatory to use only dry ether and not moist ether . The reason is that the moist ether contains water molecules and we all are aware that sodium reacts violently with water so the reaction cannot be done in moist condition.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




