
Which of the following are isoelectronic and isostructural?
\[N{{O}_{3}}^{-},\text{ }C{{O}_{3}}^{2-},\text{ }Cl{{O}_{3}}^{-},\text{ }S{{O}_{3}}\]
A. $N{{O}_{3}}^{-}$ and $C{{O}_{3}}^{2-}$
B. $S{{O}_{3}}$ and$N{{O}_{3}}^{-}$
C. $Cl{{O}_{3}}^{-}$ and $C{{O}_{3}}^{2-}$
D. $C{{O}_{3}}^{2-}$ and $S{{O}_{3}}$
Answer
590.4k+ views
Hint: Think about what both the words isoelectronic and isostructural mean. Calculate the number of electrons present in each of the given options and then find the hybridization of the central molecule to arrive at your answer.
Complete step by step solution:
The prefix ‘iso-’ means the same. Two molecules or atoms that are isoelectronic will have the same number of electrons present; add the number of electrons on each of the individual atoms and then manage the charge to get the number of electrons that are actually present. The ions and molecules that will turn out to have the same number of electrons will be considered as isoelectronic.
To see whether two molecules are isostructural or not, check the number of atoms present in that molecule and then calculate the hybridization of the central atom to see the way in which the other atoms are placed. The molecules that will have the same number of atoms along with the same geometry of the central atom will be known as isostructural.
To solve this question, let us first calculate the number of electrons present in each of the given options. The general formula to calculate the number of electrons in a molecule that has 2 types of atoms is:
\[\begin{align}
& \text{No}\text{. of electrons = }(\text{ }\!\!\#\!\!\text{ of atoms of atom 1 }\times \text{ atomic number of atom 1})+ \\
& (\text{ }\!\!\#\!\!\text{ of atoms of atom 2 }\times \text{ atomic number of atom 2})-(\text{charge on ion}) \\
\end{align}\]
- Number of electrons in $N{{O}_{3}}^{-}$: (atom 1 = N, atom 2 = O)
\[\begin{align}
& \text{No}\text{. of electrons in }N{{O}_{3}}^{-}\text{ = }(1\times 7)+(3\times 8)-(-1) \\
& \text{No}\text{. of electrons in }N{{O}_{3}}^{-}\text{ = }32{{e}^{-}} \\
\end{align}\]
- Number of electrons in $C{{O}_{3}}^{2-}$: (atom 1 = C, atom 2 = O)\[\begin{align}
& \text{No}\text{. of electrons in }C{{O}_{3}}^{2-}=(1\times 6)+(3\times 8)-(-2) \\
& \text{No}\text{. of electrons in }C{{O}_{3}}^{2-}=32{{e}^{-}} \\
\end{align}\]
- Number of electrons in $Cl{{O}_{3}}^{-}$: (atom 1 = Cl, atom 2 = O)
\[\begin{align}
& \text{No}\text{. of electrons in }Cl{{O}_{3}}^{-}=(1\times 17)+(3\times 8)-(-1) \\
& \text{No}\text{. of electrons in }Cl{{O}_{3}}^{-}=42{{e}^{-}} \\
\end{align}\]
- Number of electrons in $S{{O}_{3}}$: (atom 1 = S, atom 2 = O)
\[\begin{align}
& \text{No}\text{. of electrons in }S{{O}_{3}}=(1\times 16)+(3\times 8)-(0) \\
& \text{No}\text{. of electrons in }S{{O}_{3}}=40{{e}^{-}} \\
\end{align}\]
Here, we can see that the only 2 ions that have the same number of electrons are $N{{O}_{3}}^{-}$ and $C{{O}_{3}}^{2-}$. We will check their structures to verify their isostructural nature.
- In $N{{O}_{3}}^{-}$, nitrogen is $s{{p}^{2}}$ hybridized and forms a trigonal planar structure with the 3 other oxygen atoms. It has three different resonance structures. The geometry is as follows:
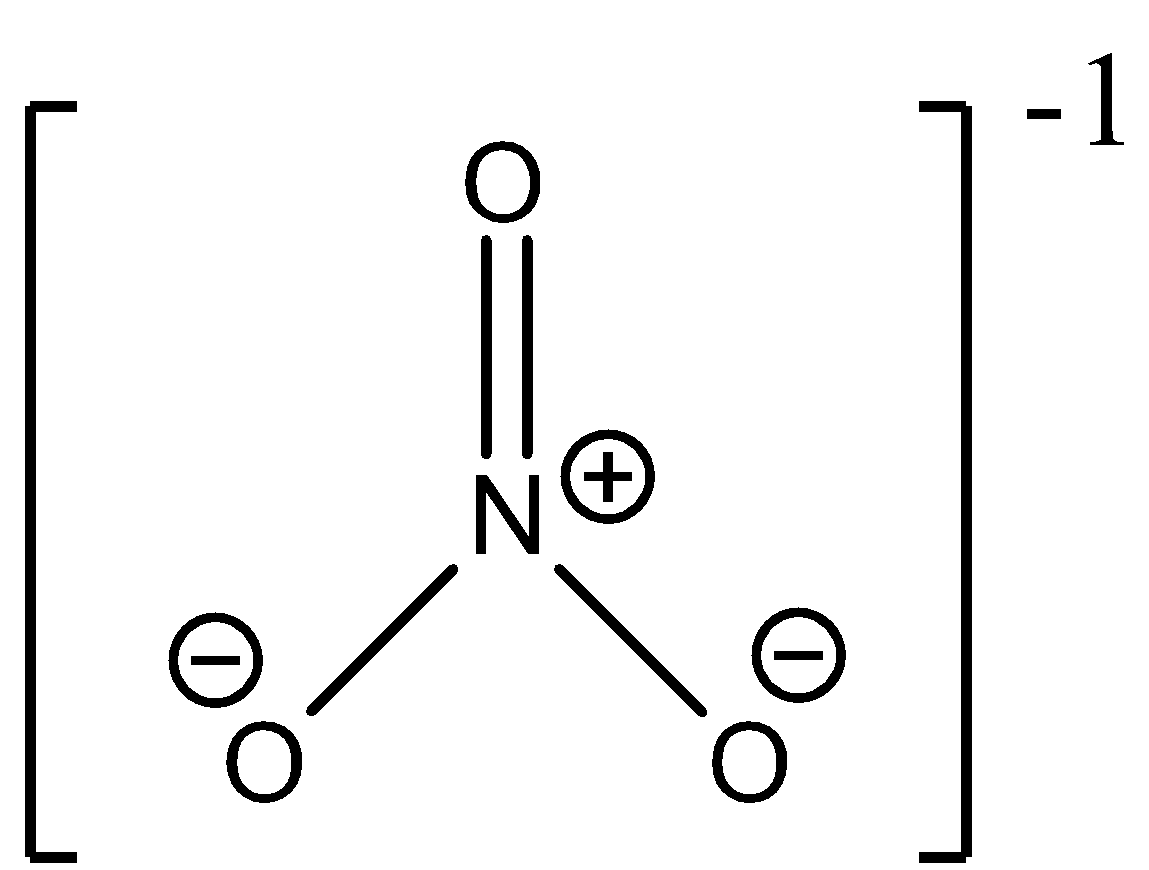
- In $C{{O}_{3}}^{2-}$, carbon too is $s{{p}^{2}}$ hybridized and forms a trigonal planar structure with the other 3 oxygen atoms. It too has three different resonating structures. The geometry is as follows:
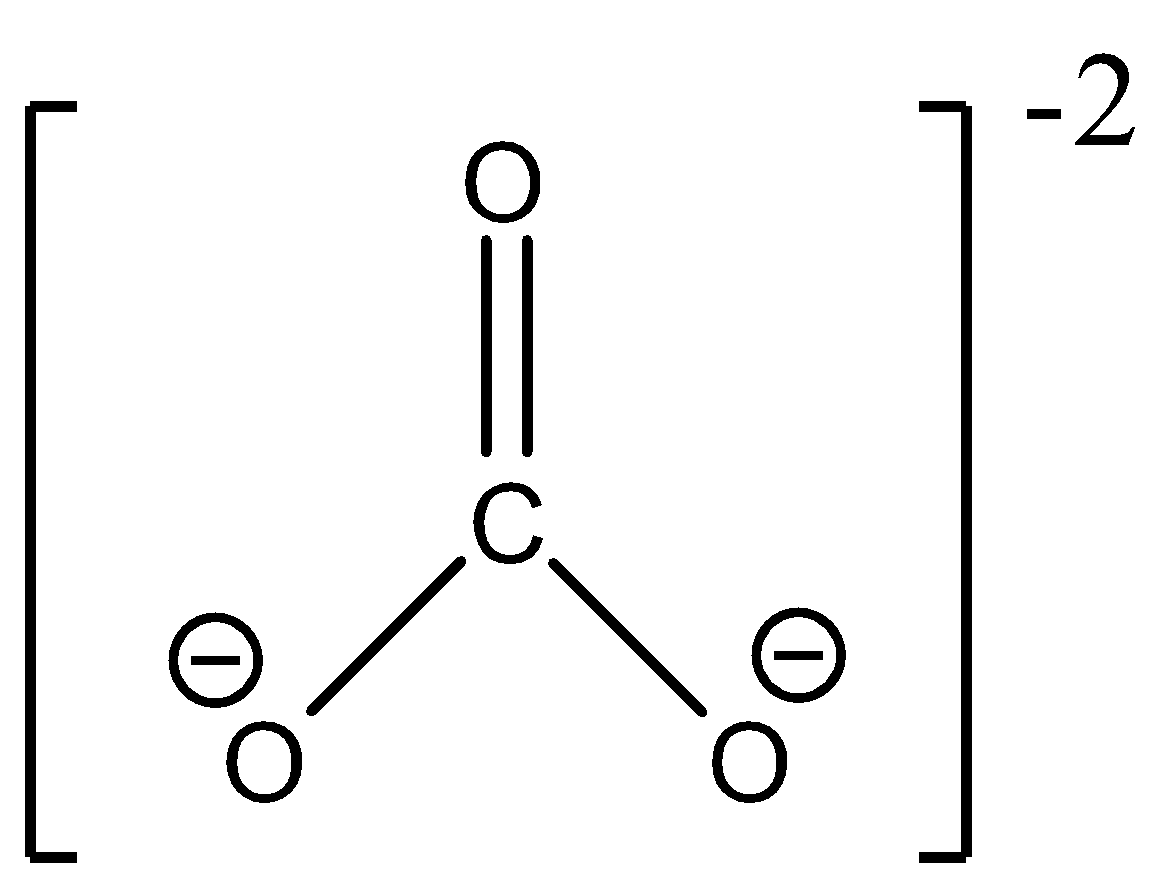
Note: We are subtracting the charge from the number of atoms in the formula to calculate the number of electrons since if the ion has a positive charge, the total number of electrons will reduce and if the ion as a negative charge then the total number of electrons will increase.
Complete step by step solution:
The prefix ‘iso-’ means the same. Two molecules or atoms that are isoelectronic will have the same number of electrons present; add the number of electrons on each of the individual atoms and then manage the charge to get the number of electrons that are actually present. The ions and molecules that will turn out to have the same number of electrons will be considered as isoelectronic.
To see whether two molecules are isostructural or not, check the number of atoms present in that molecule and then calculate the hybridization of the central atom to see the way in which the other atoms are placed. The molecules that will have the same number of atoms along with the same geometry of the central atom will be known as isostructural.
To solve this question, let us first calculate the number of electrons present in each of the given options. The general formula to calculate the number of electrons in a molecule that has 2 types of atoms is:
\[\begin{align}
& \text{No}\text{. of electrons = }(\text{ }\!\!\#\!\!\text{ of atoms of atom 1 }\times \text{ atomic number of atom 1})+ \\
& (\text{ }\!\!\#\!\!\text{ of atoms of atom 2 }\times \text{ atomic number of atom 2})-(\text{charge on ion}) \\
\end{align}\]
- Number of electrons in $N{{O}_{3}}^{-}$: (atom 1 = N, atom 2 = O)
\[\begin{align}
& \text{No}\text{. of electrons in }N{{O}_{3}}^{-}\text{ = }(1\times 7)+(3\times 8)-(-1) \\
& \text{No}\text{. of electrons in }N{{O}_{3}}^{-}\text{ = }32{{e}^{-}} \\
\end{align}\]
- Number of electrons in $C{{O}_{3}}^{2-}$: (atom 1 = C, atom 2 = O)\[\begin{align}
& \text{No}\text{. of electrons in }C{{O}_{3}}^{2-}=(1\times 6)+(3\times 8)-(-2) \\
& \text{No}\text{. of electrons in }C{{O}_{3}}^{2-}=32{{e}^{-}} \\
\end{align}\]
- Number of electrons in $Cl{{O}_{3}}^{-}$: (atom 1 = Cl, atom 2 = O)
\[\begin{align}
& \text{No}\text{. of electrons in }Cl{{O}_{3}}^{-}=(1\times 17)+(3\times 8)-(-1) \\
& \text{No}\text{. of electrons in }Cl{{O}_{3}}^{-}=42{{e}^{-}} \\
\end{align}\]
- Number of electrons in $S{{O}_{3}}$: (atom 1 = S, atom 2 = O)
\[\begin{align}
& \text{No}\text{. of electrons in }S{{O}_{3}}=(1\times 16)+(3\times 8)-(0) \\
& \text{No}\text{. of electrons in }S{{O}_{3}}=40{{e}^{-}} \\
\end{align}\]
Here, we can see that the only 2 ions that have the same number of electrons are $N{{O}_{3}}^{-}$ and $C{{O}_{3}}^{2-}$. We will check their structures to verify their isostructural nature.
- In $N{{O}_{3}}^{-}$, nitrogen is $s{{p}^{2}}$ hybridized and forms a trigonal planar structure with the 3 other oxygen atoms. It has three different resonance structures. The geometry is as follows:

- In $C{{O}_{3}}^{2-}$, carbon too is $s{{p}^{2}}$ hybridized and forms a trigonal planar structure with the other 3 oxygen atoms. It too has three different resonating structures. The geometry is as follows:

Note: We are subtracting the charge from the number of atoms in the formula to calculate the number of electrons since if the ion has a positive charge, the total number of electrons will reduce and if the ion as a negative charge then the total number of electrons will increase.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




