
Which of the following allotropes of carbon is isomorphous with crystalline
silicon?
A. Coke
B. Diamond
C. Graphite
D. Coal
Answer
578.1k+ views
Hint: The property of the elements to exhibit in more than one but in the same
The physical state is known as Allotropy. The atoms of the elements are bonded together in a
different form or manner and thus these allotropes are the modifications of these elements.
Complete step by step answer:
Coal, Graphite, Diamond and Coke are the allotropes of carbon. The carbon is the most versatile element of the periodic table. It has tetravalency and a high tendency of catenation. The ability to form the long chain of compounds is known as catenation. Due to all these reasons we see a large number of carbon compounds around us and the basis of organic compounds is only based on this very property of the carbon atom.
Among all the allotropes of carbon, Diamond has a very clear and defined shape, it has the well-defined structure. The structure of diamond is shown below:
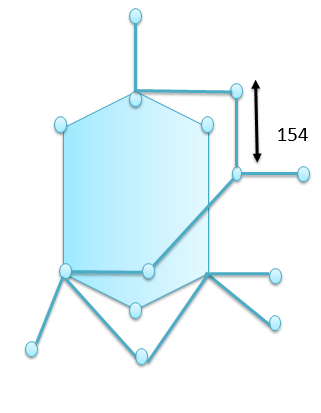
While the rest of the three coke, coal and graphite, graphite has the layered structure whereas the structure of coke and coal is not well defined since they are the combustion products of carbon obtained through different processes. Coal is obtained naturally by the decay of plants, animals and fossils, whereas the coke is the combustion product of coal.
Thus Diamond is isomorphous with the crystalline silicon since they resemble closely in their shape. The Si atom in Silicon and C atom in diamond are coordinated with 4 other atoms and they belong to the same group thus they have similar properties as well. Due to the coordination with the 4 carbon atoms they form the tetrahedral structure.
Note:
Isotopes and allotropes are two different forms both often they are confused, isotopes are the different forms of atomic structures of the same chemical element whereas allotropes are different forms of the same element.
The physical state is known as Allotropy. The atoms of the elements are bonded together in a
different form or manner and thus these allotropes are the modifications of these elements.
Complete step by step answer:
Coal, Graphite, Diamond and Coke are the allotropes of carbon. The carbon is the most versatile element of the periodic table. It has tetravalency and a high tendency of catenation. The ability to form the long chain of compounds is known as catenation. Due to all these reasons we see a large number of carbon compounds around us and the basis of organic compounds is only based on this very property of the carbon atom.
Among all the allotropes of carbon, Diamond has a very clear and defined shape, it has the well-defined structure. The structure of diamond is shown below:

While the rest of the three coke, coal and graphite, graphite has the layered structure whereas the structure of coke and coal is not well defined since they are the combustion products of carbon obtained through different processes. Coal is obtained naturally by the decay of plants, animals and fossils, whereas the coke is the combustion product of coal.
Thus Diamond is isomorphous with the crystalline silicon since they resemble closely in their shape. The Si atom in Silicon and C atom in diamond are coordinated with 4 other atoms and they belong to the same group thus they have similar properties as well. Due to the coordination with the 4 carbon atoms they form the tetrahedral structure.
Note:
Isotopes and allotropes are two different forms both often they are confused, isotopes are the different forms of atomic structures of the same chemical element whereas allotropes are different forms of the same element.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




