
Which of the following alcohol shows the fastest reaction with ${{H}^{+}}$ ?
Options-
(A)
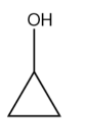
(B)
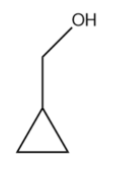
(C)
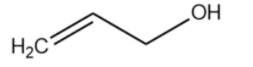
(D)
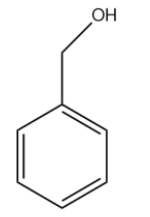




Answer
570.3k+ views
Hint: Protonate the alcohol functional group in each of the compounds. Now determine the position of carbocation formed. Identify the groups attached with the carbocation. Now understand the stabilizing factors for carbocation and determine the most stable carbocation formed. The reactivity rate is directly proportional to stability of carbocation. Thus, based on this you can determine the correct option and answer the question.
Complete answer:
We will protonate the hydroxide groups and then form carbocation for the compounds given in options as suggested in the hint.
(A)
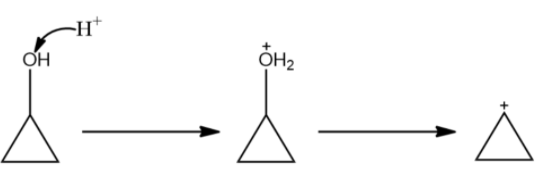
In the reaction above we see that the carbocation is formed on the carbon atom in the cyclopropyl ring. This is relatively unstable.
(B)
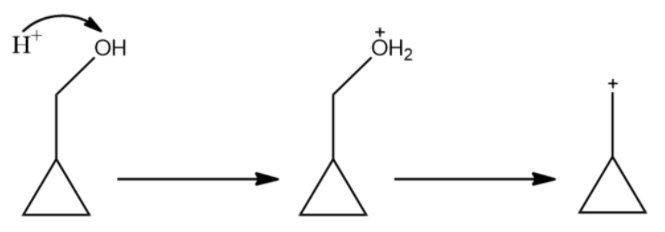
The carbocation formed in the above structure is considered to be exceptionally stable. This is due to dancing resonance observed only in cyclopropyl carbocation. Dancing resonance is a special stability mechanism that is known to increase the stability of carbocations attached directly to three membered carbon rings.
(C)
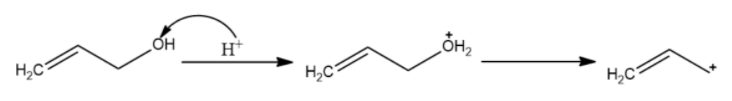
The carbocation formed is stabilised by resonance. It is an allylic carbocation.
(D)
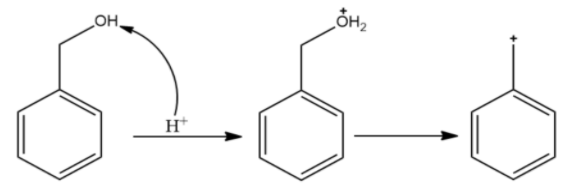
The carbocation formed is stabilised by resonance. It is a benzylic carbocation.
Based on the above statements we can conclude that the alcohol that shows fastest reaction with ${{H}^{+}}$ is,
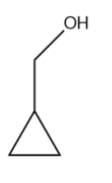
Therefore, the correct answer is option (B).
Note:
It is important to know that dancing resonance is given priority over other forms of resonance. However, this rule is applicable only in case of determining stability of carbocation.
Complete answer:
We will protonate the hydroxide groups and then form carbocation for the compounds given in options as suggested in the hint.
(A)

In the reaction above we see that the carbocation is formed on the carbon atom in the cyclopropyl ring. This is relatively unstable.
(B)

The carbocation formed in the above structure is considered to be exceptionally stable. This is due to dancing resonance observed only in cyclopropyl carbocation. Dancing resonance is a special stability mechanism that is known to increase the stability of carbocations attached directly to three membered carbon rings.
(C)

The carbocation formed is stabilised by resonance. It is an allylic carbocation.
(D)

The carbocation formed is stabilised by resonance. It is a benzylic carbocation.
Based on the above statements we can conclude that the alcohol that shows fastest reaction with ${{H}^{+}}$ is,

Therefore, the correct answer is option (B).
Note:
It is important to know that dancing resonance is given priority over other forms of resonance. However, this rule is applicable only in case of determining stability of carbocation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




